Prognostic significance of BLK expression in R-CHOP treated diffuse large B-cell lymphoma
Article information
Abstract
Background
The aim of the present study was to evaluate the prognostic significance of B-cell lymphocyte kinase (BLK) expression for survival outcomes in diffuse large B-cell lymphoma (DLBCL) patients treated with R-CHOP.
Methods
We retrospectively analyzed the medical records of 89 patients from two tertiary referral hospitals. The expression of BLK, SYK, and CDK1 were evaluated in a semi-quantitative method using an H-score, and the proportions of BCL2 and C-MYC were evaluated.
Results
A total of 89 patients received R-CHOP chemotherapy as a first-line chemotherapy. The expression rates of BLK in tumor cells was 39.2% (n = 34). BLK expression status was not significantly associated with clinical variables; however, BLK expression in tumor cells was significantly associated with the expression of both C-MYC and BCL2 (p = .003). With a median follow-up of 60.4 months, patients with BLK expression had significantly lower 5-year progression-free survival (PFS) and overall survival rates (49.8% and 60.9%, respectively) than patients without BLK expression (77.3% and 86.7%, respectively). In multivariate analysis for PFS, BLK positivity was an independent poor prognostic factor (hazard ratio, 2.208; p = .040).
Conclusions
Here, we describe the clinicopathological features and survival outcome according to expression of BLK in DLBCL. Approximately 39% of DLBCL patients showed BLK positivity, which was associated as a predictive marker for poor prognosis in patients who received R-CHOP chemotherapy.
Rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) is a standard first-line treatment for diffuse large B-cell lymphoma (DLBCL). DLBCL is an aggressive lymphoid neoplasm with heterogeneous clinical behavior that shows significantly improved survival outcomes with R-CHOP treatment [1,2]. While the international prognostic index (IPI) is useful in clinical practice, it does not fully reflect the biologic spectrum of DLBCL [3]. Adverse biological features have been investigated based on treatment outcomes in patients with DLBCL. The C-MYC/BCL2 dual expresser status and a higher rate of genetic abnormalities have been shown to contribute to the activated B-cell like (ABC) phenotype of the cell-of-origin classification in DLBCL [4,5]. However, a conclusive determination has not been established, and other biological factors are under investigation.
B-cell lymphocyte kinase (BLK) is one of the Src-family protein tyrosine kinases (SFKs) specifically expressed in B-lineage cells, thymocytes, pancreatic β-cells, and epithelial carcinoma cells [6–8]. The main BLK expression is in B cells, specifically from pro-B to B cells. Expression is diminished during development into plasma cells and is involved in the proliferation and differentiation of B cells [9,10]. B-cell receptor (BCR) formation is a principal developmental checkpoint where several Src-related kinases play redundant roles during B-cell differentiation, and the Src-related kinase BLK appears to effect functions associated with BCRs. Expression of active BLK in B cells results in a responsiveness of B progenitor cells to cytokines and leads to proliferation of these cells [11]. Spleen tyrosine kinase (SYK) and BLK kinase are recruited when BCR signaling is initiated by phosphorylation of CD79A and CD79B [12]. SYK-dependent tonic BCR signaling is an important and potentially targetable survival pathway in germinal center B cell-like (GCB) DLBCL, and the chronic active BCR signaling pathway is important in the ABC subtype of DLBCL [13,14]. Therefore, BLK expression seems to be related with DLBCL prognosis. The prognostic significance of SFK expression in DLBCL is poorly characterized.
The aim of the present study was to evaluate the prognostic significance of BLK expression for survival outcomes, including progression-free survival (PFS) and overall survival (OS), by analyzing DLBCL patients treated with R-CHOP.
MATERIALS AND METHODS
Study population
In this retrospective study, histological and immunohistochemical data were reviewed from 89 consecutive patients who were newly diagnosed with DLBCL treated with R-CHOP as a first-line chemotherapy at two of the institutions participating in the Consortium for Improving Survival of Lymphoma (CISL) study group, which is comprised of 64 centers [15]. Data from the electric medical records of 61 patients who were diagnosed in Ulsan University Hospital from 2006 to 2016 and 28 patients who were diagnosed in Kangbuk Samsung Hospital from 2004 to 2015 were analyzed. Demographic features, performance status, stage, serum lactate dehydrogenase (LDH), distant involvement sites, nodal sites, and IPI [16] were recorded. The patients were 19 years of age or older at the time of diagnosis and had no previous chemotherapy or history of other malignancy. Routine follow-up computed tomography scans were performed every three months for the first 2 years, every 6 months for the following three years, or as clinically indicated. Patients with archived paraffin-embedded tumor tissues and available follow-up data were enrolled in this study. Pathologists made the diagnosis of DLBCL at each institution, and centralized pathological review of DLBCL was conducted independently for each pathology department. The two pathologists were blind from clinical data at the time of review.
Evaluation of immunohistochemistry
Analysis of immunohistochemical staining was performed as previously described [17,18]. Establishing tissue microarray (TMA) blocks with 2 mm diameter was used in immunohistochemical staining and interpretation. Immunohistochemical staining was performed with automated immunostaining equipment (Bond Primary Antibody Diluent, Leica, Nussloch, Denmark). BLK (1:100, Abcam, Cambridge, UK), SYK (1:400, Abcam), CDK1 (1:300, Abcam), BCL2 (1:300, DAKO, Glostrup, Denmark), and C-MYC (1:100, Cell Marque, Rocklin, CA, USA) antibodies were incubated with samples for 1 hour. TMA slides were scanned using a Hamamatsu NanoZoomer XR Digital Pathology slide scanner at × 40 magnification. Digital images of the slides were analyzed using QuPath (v.0.3.2., University of Edinburgh, Scotland), an open-source pathology and bioimaging software. Evaluation of immunohistochemical staining was evaluated using a semi-quantitative method for BLK, SYK, and CDK1 using an H-score by QuPath [19], and only proportions of BCL2 and C-MYC were estimated manually. Ki-67 labeling index was counted using QuPath. Nuclear or cytoplasmic localization was determined and was assigned positive by the following predetermined criteria: H-score positivity in the cytoplasm of more than 2.5 for BLK, which is a median value in our study [9], H-score of ≥ 30 in the cytoplasm or nucleus for SYK [20], H-score of ≥ 10 in the cytoplasm or nucleus for CDK1 [21,22], ≥ 50% positivity in the cytoplasm for BCL2, and ≥ 40% positivity in the nucleus for C-MYC were regarded positive [12]. Representative images are shown in Fig. 1.
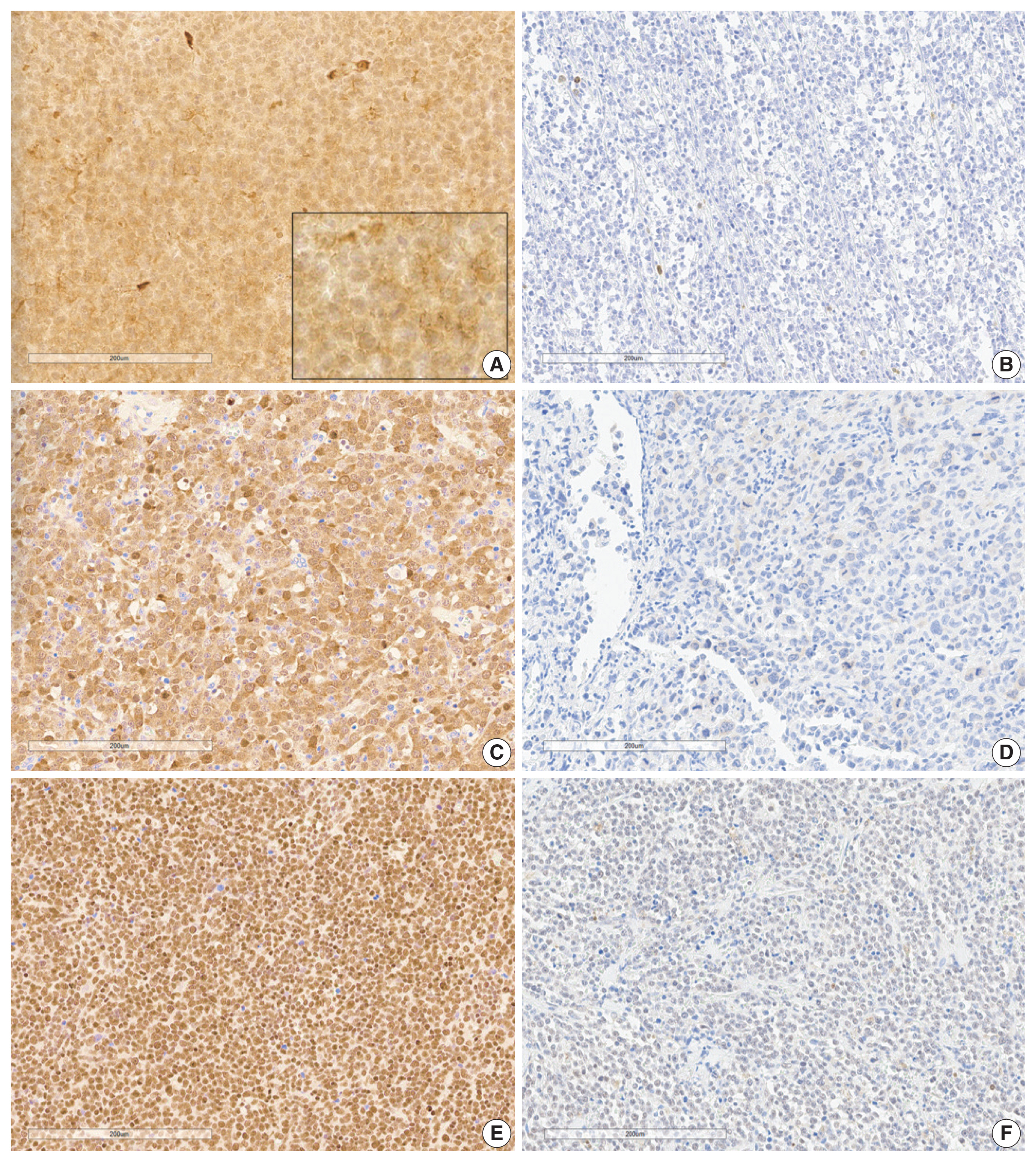
B-cell lymphocyte kinase (BLK), CDK1, and SYK expression in diffuse large B-cell lymphoma tissues. (A) Positivity of BLK expression (Inlet box is × 400 magnification). (B) Negativity of BLK expression. (C) Positivity of CDK1 expression. (D) Negativity of CDK1 expression. (E) Positivity of SYK expression. (F) Negativity of SYK expression.
Statistics
Each of the clinicopathological factors was analyzed according to their protein expression with the Pearson’s chi-square association, Fisher exact, or Student’s t tests. PFS was defined as the time from the diagnosis to the time of events such as the documented progression or recurrence of DLBCL, or death prior to progression or recurrence of DLBCL. OS was defined as the time from the diagnosis to the time of death from any cause. Patients still alive during the duration of the study were censored at the last known date of contact. The Kaplan-Meier method and log-rank test were used to evaluate the relationship between protein expression and survival results and survival curves were created. Cox regression analysis was carried out by a multivariate analysis to estimate the OS and recurrence risk ratio with a 95% confidence interval. A p-value was considered statistically significant if less than .05. Data were analyzed using the statistical package SPSS ver. 24.0 (IBM Corp., Armonk, NY, USA).
RESULTS
Study patients
This study included 46 (51.7%) male and 43 female patients (median age, 60 years; age range, 24 to 87 years). As primary treatment, all patients received R-CHOP chemotherapy. A total of 23 patients (25.8%) experienced lymphoma recurrence or progression during R-CHOP chemotherapy. Of the 23 patients, 18 (78.2%) received second-line chemotherapy, and 12 (52.2%) underwent high-dose chemotherapy followed by autologous stem cell transplantation. Supportive care was provided based on individual institutional policy.
Patient characteristics
The clinical characteristics of the 89 patients and the correlations between clinical variables according to BLK expression are summarized in Table 1. Representative immunohistochemistry images for BLK, CDK1, and SYK in DLBCL are shown in Fig. 1. The percentage of patients with positive BLK expression in tumor cells was 39.2% (n = 34). BLK expression status was not significantly associated with clinical variables. BLK positivity had a tendency toward Eastern Collaborative Oncology Group performance status (ECOG PS) 2–4, elevated serum LDH, and stage 3 or 4 compared to BLK negativity (p = .133, p = .430, and p = .173, respectively). The Ki-67 labeling index was higher in the BLK positive group, although it was not statistically significant (p = .094). Patients with BLK expression in tumor cells were more likely to have C-MYC expression (p = .057). BLK positivity was significantly associated with double expression (DE) of C-MYC and BCL2 (p = .003). Both positive group of BLK and DE of C-MYC and BCL2 was 14.6% (n = 13), and both negative group of BLK and DE of C-MYC and BCL2 was 44.1% (n = 49. p = .003).
Survival outcomes and prognostic factors
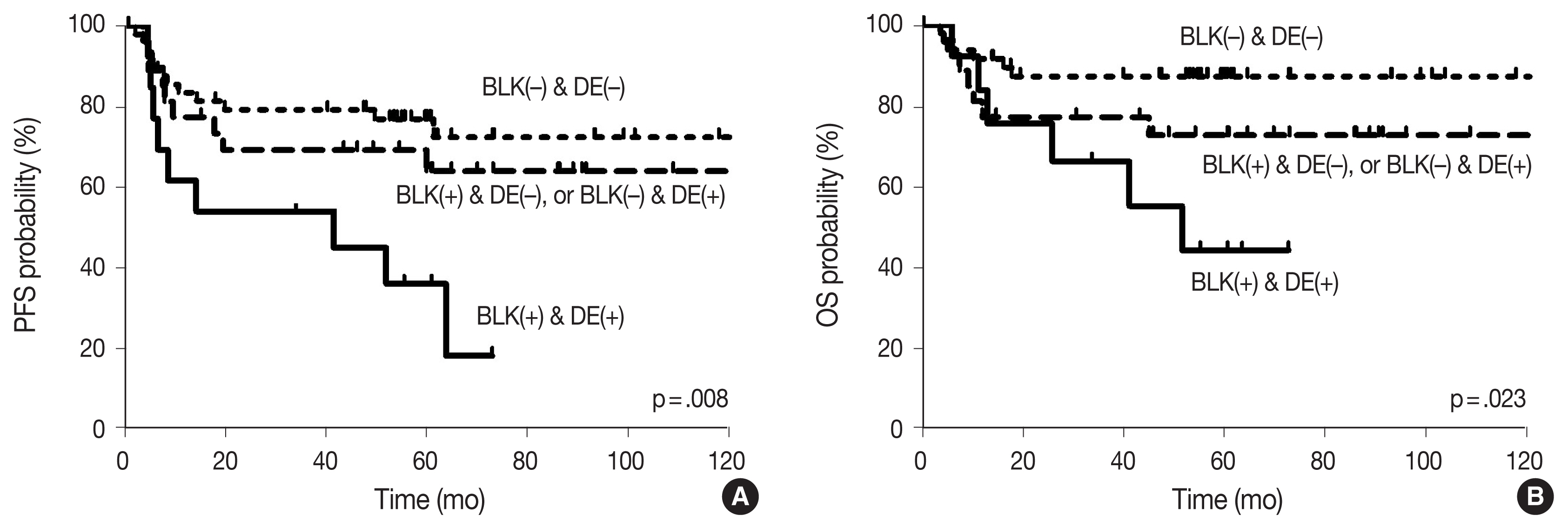
With the median follow-up time of 60.4 months, clinical variables including age ≥ 60 years, ECOG PS 2–4, elevated serum LDH, stage 3 or 4, and CDK1 positivity were significantly associated with lower PFS, and ECOG PS 2–4, elevated serum LDH, and stage 3 or 4 were significantly associated with lower OS in DLBCL patients who received R-CHOP chemotherapy as a front-line treatment (Table 2). The survival outcomes according to BLK expression are shown in Fig. 2. Patients with BLK expression had significantly lower 5-year PFS and OS rates (49.8% and 60.9%, respectively) than patients without BLK expression (77.3% and 86.7%, respectively). In multivariate analysis for PFS, BLK positivity was independently associated with shorter PFS (hazard ratio [HR], 2.208; 95% confidence interval [CI], 1.036 to 4.708; p = .040). Both positive group of BLK and DE of C-MYC and BCL2 showed significant poorer PFS and OS than the others (p = .008 and p = .023, respectively) (Fig. 3). In multivariate analysis for OS, BLK positivity was not a poor prognostic factor (HR, 2.602; 95% CI, 0.994 to 6.815; p = .052).
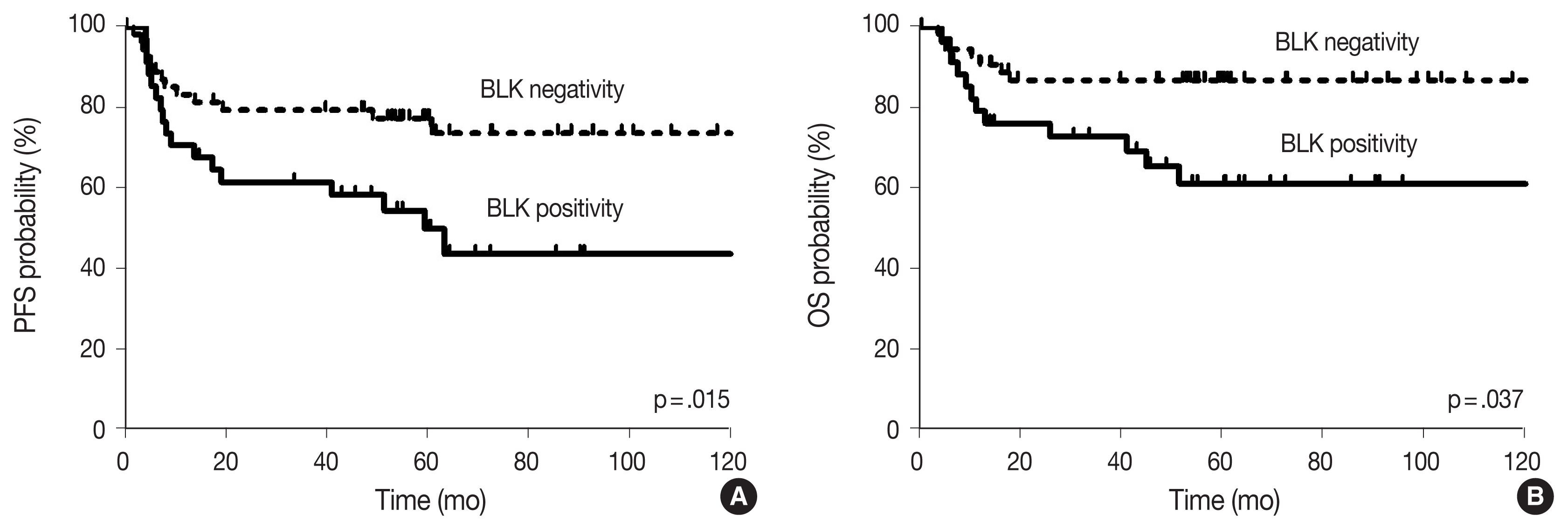
Survival outcomes according to B-cell lymphocyte kinase (BLK) expression: (A) progression-free survival (PFS) and (B) overall survival (OS).
DISCUSSION
In this study, we demonstrated BLK positivity (39.2%) in DLBCL. Although BLK expression status was not significantly associated with clinical variables such as ECOG PS 2–4, elevated serum LDH, or stage 3 or 4, BLK positivity was significantly associated with DE of C-MYC and BCL2. In the analysis of survival outcomes, BLK expression in DLBCL patients who were treated with R-CHOP as a first-line chemotherapy showed shorter PFS.
The Src-related kinase BLK is known to effect functions associated with BCRs and is principal during B lymphoid ontogeny. It is located on chromosome 8p23.1. Montero-Ruiz et al. [23] demonstrated that BLK expression has implications in childhood acute lymphoblastic leukemia. Moreover, one report showed that malignant T cells in many cutaneous T-cell lymphoma patients displayed ectopic expression of BLK, demonstrating that BLK promotes the proliferation of malignant T cells [24]. In this respect, BLK expression may have a role in lymphoma progression and may be expressed on B cell lymphomas. Our findings show that about half of DLBCL patients had BLK expression in this study cohort. Taken together, these findings suggest that BLK is a potential therapeutic target in DLBCL for SFK inhibitors. These drugs could be attractive novel candidates for the treatment of DLBLC patients expressing BLK.
Since BLK is a novel biomarker in DLBCL, we needed to establish a cutoff value. We estimated the H-score using QuPath, an open-source quantitative pathology and bioimaging software. The median H-score of 2.5 was selected; however, sampling errors could have occurred regarding tumor heterogeneity, biasing representativeness of the tumor biology. In addition, a few slides were of poor quality because they were made in the microarray. Most of the BLK positive staining was localized in the cytoplasm and membrane with little nuclear staining observed, which might be explained by the small size of the microarray wells. In 1993, David-Pfeuty et al. suggested myristoylation prevents unregulated nuclear transport of proteins in Src [25]. And further, La Roux et al. found a myristoil-binding site in the SH3 domain [25]. In a study by Urciuoli et al., a close relationship among Src nuclear localization, expression of N-myristoyltransferase, and prognosis such as OS in osteosarcoma has been observed [25]. The nuclear localization of Scr in hormone-positive breast cancer is known to be related to lower aggressiveness leading to improved prognosis. However, the localization of BLK has only been found in the membrane or cytoplasm to date [25]. Whether and how it can be localized to the nucleus, which means it is not an artifact, and what role it plays with its association to prognosis should be revealed further. As for SYK, it is reported as normally expressed in both the nucleus and cytoplasm. Zhou et al. [26] reported that other than being recruited to the membrane when BCR signaling is activated, response to oxidative stress differed according to the localization. When present in the cytoplasm, SYK increases caspase3, which is predicted to have good prognosis, whereas in the nucleus it is known to repress the activation of caspase 3.
The tyrosine residues of immunoreceptor tyrosine-based activation motif, which is located in the cytoplasmic tail of CD79a (Igα) and CD79b (Igβ), are phosphorylated by SFK, leading to the recruitment of SYK via the interaction with SH2 domains in SFKs [8]. SYK is recruited to the perimembrane to be phosphorylated by SFK, auto-phosphorylated, and subsequently activated. It is activated in 44% of human DLBCL tissues. SYK is the key factor in initiating the pre-BCR signaling cascade, which leads to B cell activation and B-T cell interaction [27]. The oncogenic function of BLK seems to be dependent on the cellular context [28]. More SYK expression was observed in the BLK positive group in univariable analysis, although it was not statistically significant (p = .118). They were also not associated with prognosis (p = .281 and p = .276, PFS and OS, respectively).
Cheung et al. demonstrated that PLCγ2 and AKT, as opposed to ERK, connected SYK to cell proliferation and when blocked, the G1 to S transition was blocked leading to cell cycle arrest. The study did not find a correlation between SYK inhibition and cell-of-origin subtypes [29]. However, Davis et al. [30] showed knockdown of proximal BCR subunits (IgM, Ig, CD79A, CD79B) and SYK induced death to ABC DLBCLs with wild type CARD11 but not in other lymphomas including the GCB subtype. AKT activity is also reported to be high in the G2/M phase, but in epithelial cells [31]. The different consequences of the BCR pathway between ABC and GCB DLBCL has been reported, where both AKT and nuclear factor κB are activated in the ABC group and only AKT is activated in GCB group depending on the site of tyrosine phosphorylation on SYK [14]. Wang et al. [32] showed expression of p-AKT nuclear expression of 70% or more was observed in 24.3% of de novo DLBCL patients treated with R-CHOP. It was also associated with poor PFS but not survival. Moreover, this overexpression was associated with DE [32]. In our study, BLK and SYK status were not significantly different between the subtypes, with BLK status slightly higher in the non-GCB group and SYK status slightly higher in the GCB group (p = .832 and p = .781, respectively).
Although it is well known that dual expression of C-MYC and BCL2 in DLBCL is associated with poorer outcomes [4,33], the prognosis with respect to BLK expression as a Src family kinase in DLBCL has not been determined. Our finding in DLBCL shows that BLK positivity is a poor prognostic factor for PFS, and trends toward poorer OS. Moreover, both positive BLK groups and DE of C-MYC and BCL2 showed significantly worse PFS and OS than others. It suggested that expression of BLK in our case may have contributed to the proliferation of lymphoma cells promoting tumor growth.
Since DE status was different statistically based on BLK expression (p = .003) in univariable analysis, it was not included in multivariable Cox regression analysis. In a study by Wang et al. [12], MYC-positive DLBCL had higher levels of pSYK and pBLK, which are their activated forms. They also revealed that MYC overexpression might be a mechanism for resistance to the BCR inhibitor, ibrutinib, and may help to identify potential therapeutic candidates [12]. Bogusz et al. [18] concluded that DE DLBCL shows enhanced BCR pathway signaling compared to non-DE DLBCL based on phosphomarker expression and the exact mechanisms linking BCR signaling to MYC expression have yet to be revealed. However, it has been shown that MYC is a key downstream BCR effector, and its overexpression can rescue the absence of BCR activity in some B cells. The upregulation of MYC has been observed in ibrutinib-resistant mantle cell lymphoma cell lines and this was reversed by inhibiting HSP90. Recently, ibrutinib has been approved as a co-treatment with venetoclax, a BH3 mimetic that inhibits BCL2 for chronic lymphocytic leukemia since ibrutinib induces BCL2 expression [34]. Kuo et al. [35] demonstrated that ibrutinib and the BCL-2 inhibitor ABT-199 showed a synergistic effect on both ABC and GCB DLBCL cell lines. It can be inferred that BCR signaling increases MYC expression, MYC and BCL2 can be a mechanism of resistance when targeting BCR signaling pathways and contribute to poor prognosis.
Lastly, CDK1 is a cell cycle regulator and Wolowiec et al. [36] showed it is increased in acute lymphoblastic leukemia and diffuse lymphoma only in proliferative cell cycle phases including S, G2, and M. The CDC2 gene, which encodes cdc2/cdk1, is amplified only in a subset of DLBCL, but the cdc2/cdk1 protein was detected in the majority of DLBCL patients and cyclin B1 expression was reported to be related to poor prognosis, implying that high levels of cdc2/cdk1 may predict aggressiveness in DLBCL [37]. Moreover, higher CDC2 amplification was identified as related with primary aggressive and recurrent DLBCL or cause of chemoresistance [38]. Other mechanisms that may have contributed to CDK1 overexpression, which is revealed to be a predictive factor, should be revealed further. In other neoplasms, CDK1 is observed to be a prognostic factor in oral squamous cell carcinoma, and Epstein-Barr virus (EBV)–encoded oncoprotein latent membrane protein 1 inducing CDK1 resulting in partial upregulation of the anti-apoptotic factor survivin in nasal natural killer/T-cell lymphoma [21,22]. Similarly, CDK1, cyclin B1, and survivin expression were shown to be increased in post-transplant EBV-lymphoproliferative diseases [39]. CDK1 was associated with PFS but not OS in univariable analyses (p = .039, and p = .507, PFS and OS, respectively).
This study has several limitations inherent to retrospective analyses and relatively small sample size. Further studies would help to validate these findings and bring additional information. On the other hand, this report is among the few of which had a relatively long follow-up duration and describe a group of patients undergoing uniform first-line chemotherapy from the CISL group in Korea.
In conclusion, the therapeutic strategy of specific signaling inhibition could be a pivotal study field in lymphoma treatment. However, the targeting for SFK in DLBCL is under investigation. The results of the current study reveal that BLK is expressed in approximately half of DLBCL cases. Moreover, BLK expression is associated with poorer survival outcomes. These findings provide important information to advance the establishment of treatment strategies for DLBCL.
Notes
Ethics Statement
All data collection and analysis were performed in accordance with the Helsinki Declaration. This study was approved by the Institutional Review Board (IRB) of Ulsan University Hospital (IRB No. 2017-06-24-001). Formal written informed consent was not required with a waiver by the Clinical Ethics Committee.
Availability of Data and Material
The datasets generated or analyzed during the study are available from the corresponding author on reasonable request.
Code Availability
Not applicable.
Conflicts of Interest
The authors declare that they have no potential conflicts of interest.
Author Contributions
Conceptualization: HJC, SWC, JCJ. Formal analysis: HJC, MK, JCJ. Funding acquisition: HJC. Investigation: HJC, SWC, JCJ. Resources: YJL, YC, MK, HJK, JEK, SO, SWC, HJC, JCJ. Visualization: HJC, SWC. Writing—original draft: SC, HJC, JCJ. Writing— review & editing: SC, YJL, YC, MK, HJK, JEK, SO, SWC, HJC, JCJ.
Funding Statement
This work was funded by Ulsan University Hospital (Biomedical Research Center Promotion Fund; UUH-2017-03).