Current status of cytopathology practice in Korea: impact of the coronavirus pandemic on cytopathology practice
Article information
Abstract
Background
The Continuous Quality Improvement program for cytopathology in 2020 was completed during the coronavirus pandemic. In this study, we report the result of the quality improvement program.
Methods
Data related to cytopathology practice from each institute were collected and processed at the web-based portal. The proficiency test was conducted using glass slides and whole-slide images (WSIs). Evaluation of the adequacy of gynecology (GYN) slides from each institution and submission of case glass slides and WSIs for the next quality improvement program were performed.
Results
A total of 214 institutions participated in the annual cytopathology survey in 2020. The number of entire cytopathology specimens was 8,220,650, a reduction of 19.0% from the 10,111,755 specimens evaluated in 2019. Notably, the number of respiratory cytopathology specimens, including sputum and bronchial washing/ brushing significantly decreased by 86.9% from 2019, which could be attributed to the global pandemic of coronavirus disease. The ratio of cases with atypical squamous cells to squamous intraepithelial lesions was 4.10. All participating institutions passed the proficiency test and the evaluation of adequacy of GYN slides.
Conclusions
Through the Continuous Quality Improvement program, the effect of coronavirus disease 2019 pandemic, manifesting with a reduction in the number of cytologic examinations, especially in respiratory-related specimen has been identified. The Continuous Quality Improvement Program of the Korean Society for Cytopathology can serve as the gold standard to evaluate the current status of cytopathology practice in Korea.
The Continuous Quality Improvement program (CQI) for cytopathology laboratories was first started in 1995 by the Committee of Quality Improvement of the Korean Society for Cytopathology (CQIKSC) and has significantly contributed to the quality control and improvement of cytopathologic examination over the last two decades [1-4]. As the number of cytopathologic examinations increased from 2.8 million in 2004 to over 10 million in 2018, the number of participating institutions increased from 100 in 1996 to 214 in 2020 [2]. Despite the exponential increase in cytopathologic examinations in Korea, sample adequacy has been relatively well controlled as well as other quality parameters such as the atypical squamous cells/squamous intraepithelial lesion (ASC/SIL) ratio [2]. Meanwhile, the ratio of discordant cases with major clinical impact (category C) in the cytology-histology correlation review of gynecologic samples decreased from 1.59% in 2003 to 0.52% in 2018 [2]. In conjunction with the National Cancer Screening Program started in 1999, CQI successfully contributed to a reduction in the incidence of cervical cancers by improving the quality of cytopathologic examinations in Korea [2,3,5].
However, the coronavirus pandemic from late 2019 has had an unprecedented impact on all aspects of our lives and society and has fundamentally changed the whole landscape of cytopathology practice. Since the first patients with coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome-coronavirus 2 appeared in China in late 2019, 610 million confirmed cases were reported with this ongoing pandemic worldwide and over 6.5 million people were have been estimated to have died of this devastating disease until September 2022 [6]. In a recent systematic review, COVID-19 pandemic was reported to have caused large reductions in many healthcare services, major methodological changes, and the emergence of new technologies such as telemedicine and digital pathology because of measures such as lockdowns and stay-at-home orders during the pandemic period [7]. In cytopathology practice, the pandemic appears to have caused a large reduction in the number of respiratory tract samples. However, there is limited evidence and data representing the actual impact of COVID-19 on cytopathology practice. Moreover, with clinical implementation of digital cytopathology and telecytopathology gaining more attention from the cytopathology field, the availability and safety of these new technologies is also being actively questioned and tested for proper validation evidence.
During the pandemic period, CQIKSC newly introduced digital cytopathology in CQI in parallel with conventional methods and conducted an annual survey of the statistics for gynecology (GYN) cytologic exams, which included assessments of overall statistics, statistics on the diagnostic category of GYN exams, and inadequacy rates, cytology-histology correlation reviews of gynecologic samples, evaluation of the number of discordant cases, proficiency test using five glass slides and digital cytopathology with whole-slide images (WSIs), sample adequacy evaluations using both glass and digital slides, and a submission of candidate glass and digital slides for the next proficiency test. Here, we present the results of the nationwide survey of the annual statistics for cytologic exams in 2020 and the proficiency test for 2021.
MATERIALS AND METHODS
Outline of quality improvement program by the Committee of Quality Improvement of the Korean Society for Cytopathology
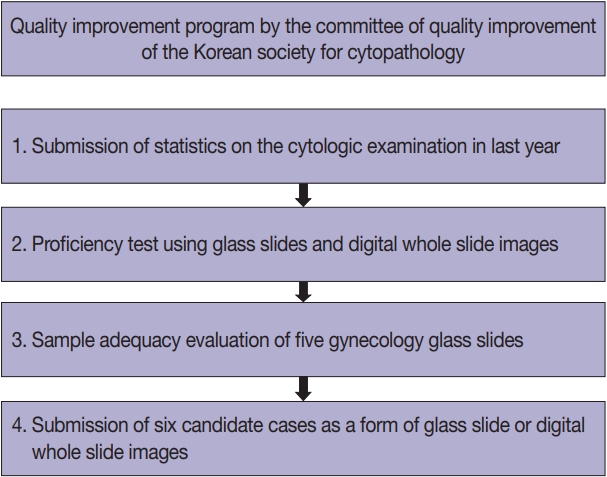
The annual CQI included the following steps: 1, collection of statistics on the cytologic examinations performed in the last year; 2, a proficiency test using five glass slides or six digital WSIs; 3, a sample adequacy evaluation of five GYN glass slides submitted by the participating institutions; and 4, submission of six candidate cases by the participating institutions to be used in the proficiency tests in the following years (Fig. 1).
Annual survey of cytopathology statistics
Internal quality control data were collected from 214 medical institutions using a portal questionnaire that included the statistical data on overall cytologic exams, the case number of GYN exams according to the diagnostic categories, the GYN sample adequacy, the cytology-histology correlation review of gynecologic sample results, and the number of discordant cases according to the discordant assessment criteria. The participating institutions were categorized into three groups: university hospitals, general hospitals, and commercial laboratories. When the cytology-histology correlation review of gynecologic sample results was evaluated, diagnostic concordance was evaluated as concordant (category O), discordant category A (minimal clinical impact), discordant category B (minor clinical impact), or discordant category C (major clinical impact). Statistical data for cytologic examinations were calculated and organized by sample categories as follows: GYN, fine-needle aspiration (FNA), and non-GYN/non-FNA sample examinations including examination using urine, body fluids, respiratory tract samples (sputum, bronchial washing, brushing, bronchioloalveolar lavage, etc.), and cerebrospinal fluid.
Proficiency test
The second part of CQI, the proficiency test, was performed in 215 medical institutions from June 7, 2021, to June 18, 2021 using a total of 540 glass slides and 30 WSIs. Five glass slides, two GYN slides, two slides of body fluid and/or urine samples, and one slide of two respiratory tract or FNA samples were sent by 108 participating institutions, while six WSIs, including two GYN slides, two body fluid or urine sample while two respiratory tract samples or FNA slides were distributed randomly to 107 participating institutions and accessed by the website (Fig. 2). The cases submitted as glass slides and WSIs were different. The cases were reviewed and confirmed by members of CQIKSC. The diagnoses submitted by the participating institutions was selected using the diagnostic template (Supplementary Tables S1–S3). The diagnostic concordance was evaluated as follows: concordant (category O), discordant category A (minimal clinical impact), discordant category B (minor clinical impact), or discordant category C (major clinical impact). If an institution received one or more category C results, it was required to undergo retesting to obtain quality assurance certification.
Sample adequacy assessment
For the sample adequacy assessment, participating institutions submitted five GYN glass slides with the consequent sample number and pathology report, including the content for sample adequacy. Sample adequacy was then reevaluated by the members of CQIKSC.
Submission of samples for the quality assurance program
For the final part of the program, each participating institution was asked to submit six glass slides (2 GYN, 2 non-GYN, and 2 FNA) with confirmed cytologic diagnoses and the corresponding histologic diagnoses from November 26, 2018, to December 7, 2018. The eligibility of the collected samples for the proficiency test was evaluated by the members of CQIKSC, and eligible samples were archived to be used in the proficiency test in the following years.
RESULTS
Participating institutions
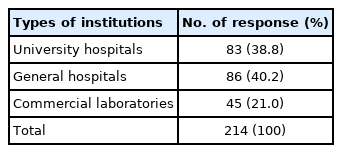
Responses were obtained from all 214 medical institutions, including 83 university hospitals (38.8%), 86 general hospitals (40.2%), and 45 commercial laboratories (21.0%) (Table 1). The total number of institutions that participated in the survey increased by six, in comparison with the number in 2019. The number of university hospitals, general hospitals, and commercial laboratories increased by three, two, and one, respectively.
Overall statistics
The total number of cytopathologic examinations performed in 2020 was 8,220,650, of which 7,068,938 (86.0%) samples were GYN samples (cervical cytology, 7,063,922; gynecologic fluid samples: ovary and endometrium, 5016) and 1,151,712 (14.0%) samples were non-gynecological.
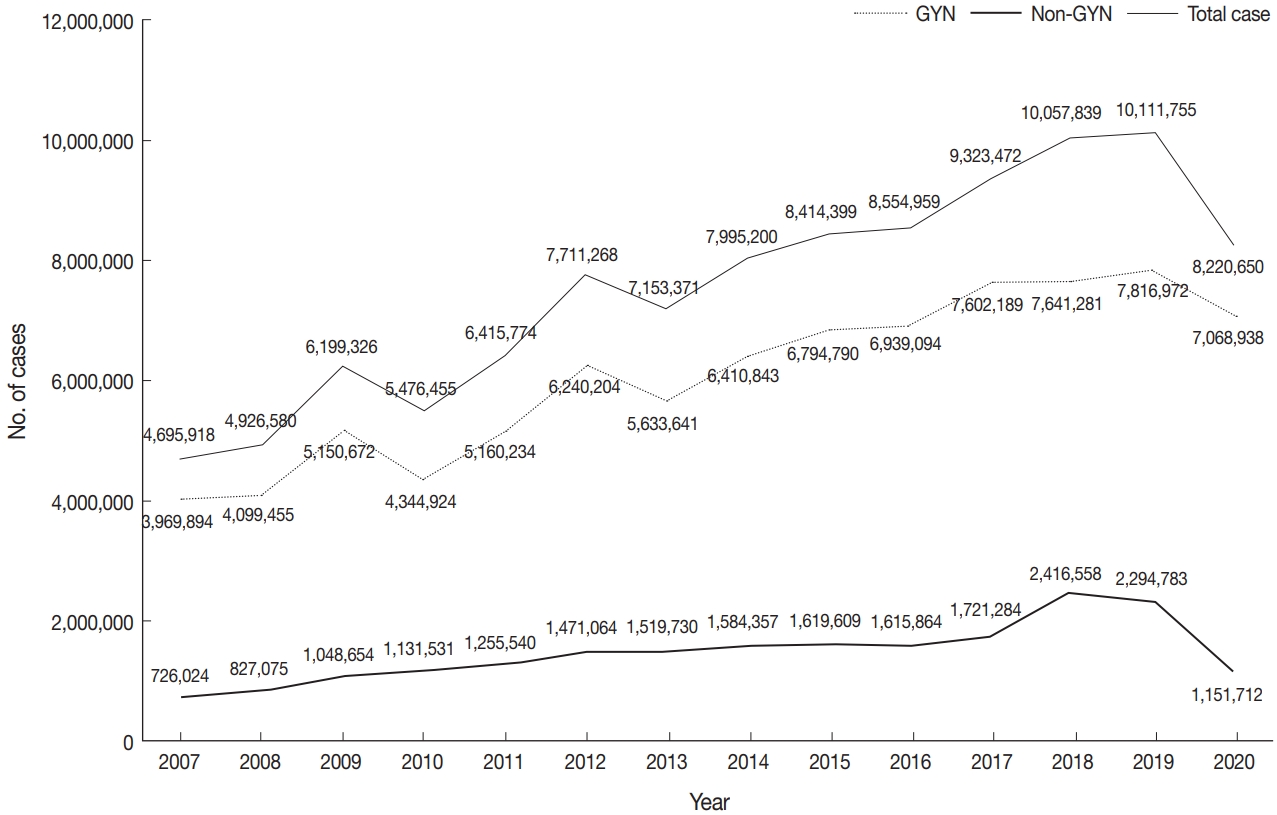
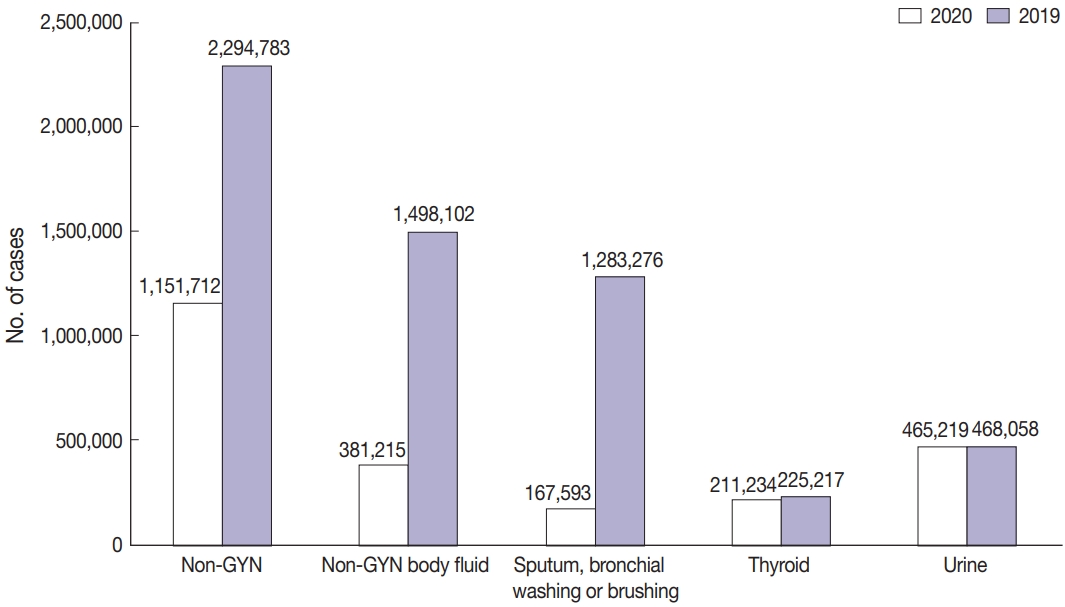
As seen in Fig. 3, the total number of cytopathologic examinations performed in Korea was increased every year, but abruptly dropped by 19% during the COVID-19 pandemic (from 10,111,755 in 2019 to 8,200,650 in 2020). In particular, the non-GYN sample number decreased significantly by 50% (from 2,294,783 in 2019 to 1,151,712 in 2020). On the other hand, the decrease in the GYN sample number was moderate, from 7,816,972 in 2019 to 7,068,938 in 2020 (about 10%).
As a result of these changes, the proportion of GYN samples in 2020 was 86%, which is an increase from before COVID-19, and the proportion of non-GYN samples was 14%, which is a reduction from that before COVID-19.
Sample types
Among the 1,151,712 non-GYN samples in 2020, the number of FNA, urine, and non-GYN body fluid samples was 305,278, 465,219, and 381,215, respectively (Fig. 4A). The FNA samples included 211,234, 5,665 and 88,379 thyroid FNA, lung FNA, endoscopic ultrasound/endobronchial ultrasound aspiration samples, and the body fluid samples consisted of 167,593, 142,804, 26,213, and 44,605 sputum, bronchial washing or brushing; body cavity fluid (pleural fluid, pericardial fluid or ascites); cerebrospinal fluid; other miscellaneous fluid samples (Fig. 4B).
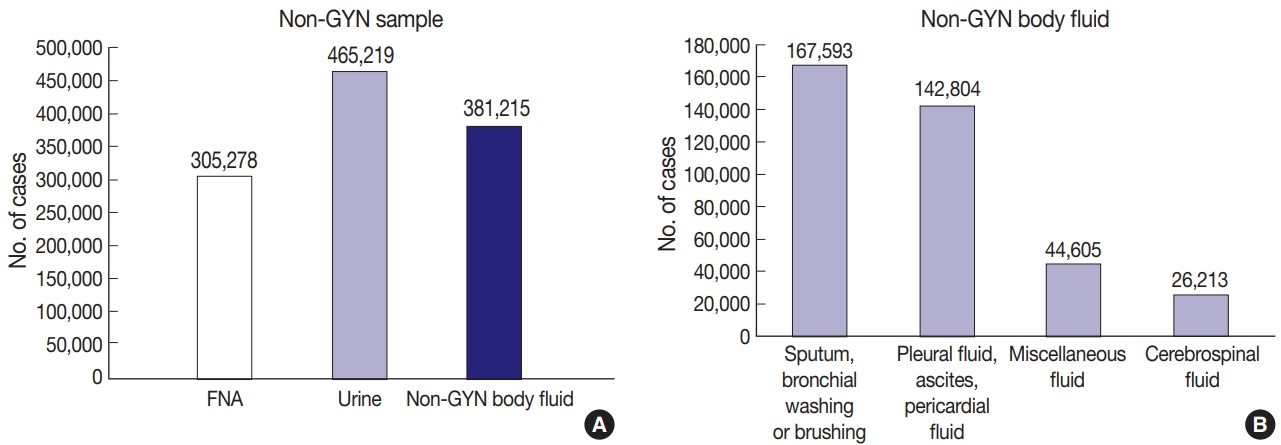
(A) The number of non-gynecologic (non-GYN) samples, according to the type of sample. (B) The number of non-GYN body fluid, according to the type of sample.
As mentioned above, the number of non-GYN samples decreased sharply by 49.8% during the COVID-19 period, of which the number of non-GYN body fluid samples decreased by 74.5% in 2020, in comparison with 2019. In particular, the decrease in sputum, bronchial washing or brushing samples was noticeable, down 86.9% from the previous year in 2020. In contrast, the number of thyroid FNA and urine samples decreased by 6.2% and 0.6%, respectively in 2020 (Fig. 5).
The total number of GYN samples obtained in 2020 was 7,068,938, and these samples were most frequently processed by commercial laboratories. In 2020, the proportion of GYN samples processed in commercial laboratories, university hospitals, and general hospitals was 83.4% (n=5,895,184), 11.0% (n=774,375), and 5.6% (n=399,379), respectively. Considering the numbers of institutions (45 commercial laboratories, 83 university hospitals and 86 general hospitals), it can be inferred that commercial laboratories process a considerable amount of GYN samples and few non-gynecological samples.
Conventional and liquid-based cytology
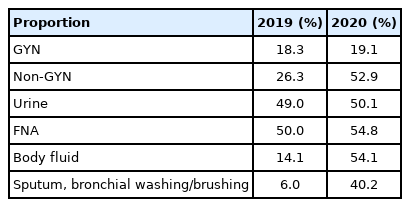
A total of 6,263,193 samples (76.2%) were prepared by conventional smear, and 1,957,457 samples (23.8%) were prepared by liquid-based cytology (LBC). The LBC method was used in 19.1% of the GYN (n=1,348,699) and 52.9% of the non-GYN samples (n=608,758), including 50.1% of the urine (n=233,216), 54.8% of the FNA (n=167,270), and 54.1% of the body fluid (n=209,044) samples (Table 2). The LBC coverage of GYN samples slightly increased from 2019 to 2020 (18.3% to 19.1%). Notably, the LBC coverage of non-GYN samples dramatically increased from 2019 to 2020 (26.3% to 52.9%) due to the high LBC ratio of body fluid samples. Surprisingly, the LBC coverage markedly increased by 40.0% for sputum, bronchial washing or brushing specimens. These results can be attributed to a decreased number of sputum smears, not an increase in LBC. The LBC coverage of GYN samples slightly increased from 2019 to 2020 in all university, general hospitals, and commercial laboratories. However, in non-GYN samples, LBC coverage for all kinds of institutions increased markedly from 2019 to 2020 (university hospitals, 53.7% to 67.9%; general hospitals, 33.9% to 57.1%; commercial laboratories, 8.33% to 28.6%). Further analysis demonstrated that an increased non-GYN LBC ratio occurred from body fluid samples with an increased LBC ratio across all types of institutions.
Distribution of GYN sample cytologic diagnoses
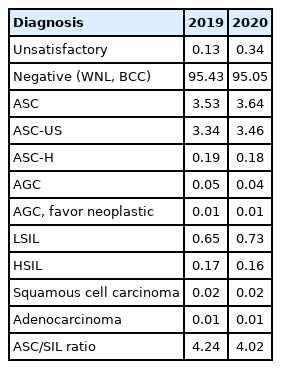
GYN sample cytologic diagnoses from each institution were collected and analyzed in Table 3. The percentage of the samples with “unsatisfactory adequacy” was 0.34% in 2020. The percentage of “negative” samples, including those within normal limits and those with benign cellular changes, was 95.05%. The percentages of “atypical squamous cells of undetermined significance” and “atypical squamous cells-cannot exclude high-grade squamous intraepithelial lesions” were 3.46% and 0.18%, respectively. The percentages of “low-grade squamous intraepithelial lesion” and “high-grade squamous intraepithelial lesion” were 0.73% and 0.16%, respectively. The ASC/SIL ratio was 4.10 in 2020. In comparison with the data for 2019, the percentage of atypical squamous cells of uncertain significance increased from 3.34% to 3.46%, and the percentage of SIL slightly increased from 0.87% to 0.89%. With regard to institutions, the ASC/SIL ratio was steadily maintained at approximately 1.81 in university hospitals and 2.71 in general hospitals. In contrast to the previously recorded continuous increasing in the ASC/SIL ratio in commercial laboratories, it slightly decreased from 5.68 in 2019 to 5.10 in 2020.
Cytology-histology correlation review of gynecologic samples
As a part of the internal quality control program, each institution was asked to compare the diagnoses of the cytologic and histologic samples of the same individual whenever possible and to document the degree of discordance between the cytologic and histologic diagnoses according to the institutional criteria. Of the 39,456 cases, 30,451 cases showed concordant results between the cytologic and histologic diagnoses (77.2%). Discordance with minimal and minor clinical impact (categories A and B) was found in 5,948 and 2,088 cases, respectively (15.1% and 5.3%). Discordance with major clinical impact (category C) was found in 378 cases (0.96%).
Adequacy of GYN samples
The percentages of GYN samples that were considered unsatisfactory or adequate in each institution were collected as a part of the annual survey. The unsatisfactory rate was 0.34% in 2020. It was 1.38% in university hospitals, 1.78% in general hospitals, and 0.10% in commercial laboratories in 2020.
Proficiency test
The rates of overall concordance (category O), discordance with minimal clinical impact (category A), discordance with minor clinical impact (category B), and discordance with major clinical impact (category C) findings were 82.0%, 13.7%, 2.6%, and 0.1%, respectively. In category C, seven cases diagnosed with malignancy were interpreted as benign, while three cases with benign disease were interpreted as malignancy.
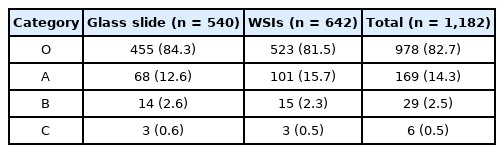
In assessments based on the source used for the proficiency test, in the 108 institutions selecting five glass slides, categories O, A, B, and C represented 84.3%, 12.6%, 2.6%, and 0.6% of the findings. On the other hand, in 107 institutions choosing six WSIs, showed categories O, A, B, and C represented 81.5%, 15.7%, 2.3%, and 0.5% of the findings (Table 4). The distribution of results in the proficiency test in relation to the source (glass slides or WSIs) was similar. Of note, the rates for categories C in both glass slides and WSIs were below 1%.
Sample adequacy assessment
CQIKSC requested five GYN glass slides with consequent numbers. A total of 197 participating institutions submitted 985 GYN slides and their reported adequacy. The sample adequacy assessments reevaluated by the members of CQIKSC and the adequacy of the institutions were concordant in all cases. For 17 institutions that were unable to submit GYN slides, sample adequacy was examined using GYN WSIs. All participating institutions passed the tests.
Submission of samples for the next quality assurance program
A total 176 participating institutions submitted 1,056 cases in the form of glass slides, while 11 institutions sent WSIs for 33 cases. Twenty-six institutions that were unable to submit samples, participated and passed the test for cytologic diagnosis using WSIs.
DISCUSSION
Four reports for the quality improvement program and its results have been published by the Korean Society for Cytopathology in the form of articles in 2008, 2017, and 2020 [1-4]. These articles showed the status of cytopathology practice in Korea, including the participating institutions, overall statistics, GYN and non-GYN samples, conventional and LBC cytology, distribution of the GYN sample cytologic diagnosis, gynecologic cytology-histology correlation review, adequacy of GYN samples, proficiency test, sample adequacy assessment, and submission of samples for the next quality assurance program. After that, the COVID-19 pandemic drastically changed the status of practice of cytopathology as well as medical practice. Therefore, a report of cytopathology practice in Korea, that reflect the impact of the COVID-19 pandemics is urgently needed.
First, the number of cytopathologic tests in 2020 was 8,200,650, a significant reduction from the number in 2019 (10,111,755 cases). According to the National Health Insurance Statistical Yearbook, the number of patients using medical institutions in 2020 dropped to 48.57 million from 49.63 million in 2019 [8]. Thus, the drop in the total number of cytologic examinations occurred due to the COVID-19 pandemics.
However, regarding the discrepancy between the cytopathologic examinations and the medical institutions, it can be reasonably speculated that cytologic tests in Korea were conducted in line with health or cancer screening programs. According to the E-national indicator issued by the Ministry of Health and Welfare, the screening rate of the National Cancer Screening Program decreased to 55.4% in 2020 from 62.2%, 2019. Furthermore, the number of cervical smear cytology specimens as a part of the National Cancer Screening Program dropped to approximately 2,756,000 from 3,141,000 [9].
The discordance rate in the cytology-histology correlation review of gynecologic samples slightly decreased in 2020 (20.54%) compared to 2019 (23.84%). The discordant cases occurred mainly with minimal clinical impact (17.84%) that was not harmful to the management of patients. The most important finding, that the rate of category C (major clinical impact) has been well controlled below 1% since 2012, indicated the usefulness of cervical cytologic examination. On the other hand, the number of submitted data points in 2020 was 39,456, only half the number of data points collected in 2018 [2]. Thus, a fine guideline on collecting, managing, and reporting data for the cytology-histology correlation review of gynecologic samples is required.
LBC offers numerous advantages over conventional smears, including advantage in interpretation, convenience, and filtering out of contaminating debris and blood [10]. Moreover, conventional smears involve a certain of workload for pathologists as well as cytotechnologists [11]. The proportion of LBC examinations in non-GYN samples has noticeably increased from 26.3% in 2019 to 52.9% in 2020. This change was not due to an increase in the absolute number of LBC examinations, but rather due to reduction in sputum smear cytology. Additional careful investigations into the health effects of the marked reduction in respiratory-related conventional smear cytology should be performed, since these results could support the usefulness of sputum cytology.
A previous study in the United States reported that the median unsatisfactory rate was 0.5% and 90% of institutions had an unsatisfactory rate below 2% [12]. The unsatisfactory rate for GYN cytology in 2020 was 0.34% across all kinds of institutions and 1.38% in university hospitals, 1.78% in general hospitals, and 0.10% in commercial laboratories. Although all institutions had unsatisfactory rate below 2%, the unsatisfactory rate for GYN cytology examinations varied substantially, according to the type of institution. In this regard, the education and the establishment of defined criteria by the Korean Society for Cytopathology would be essential.
The ASC/SIL ratio can serve as a good marker to monitor the level of certainty and specificity [13,14]. Current recommendations for the ASC/SIL ratio suggest a ratio of less than 3:1 [15]. In 2020, the ASC/SIL ratio in all institutions was 4.10. The ASC/SIL ratios of university hospitals and general hospitals were 1.81 and 2.71, respectively, i.e., <3.0. However, the ASC/SIL ratio in commercial laboratories has remained above 5.0 from 2017 to 2020. Commercial laboratories have an important position in the diagnosis of cervical cytology because they process most of the cases in Korea. However, considering the multivariable factors affecting ASC/SIL ratio, including the workload of cytopathologists and cytotechnologists, a proper working environment and optimization of other factors is essential.
The use of WSIs in cytopathology has been more difficult than in histology because of the nature of cytological investigations, including the thickness and 3D distribution of cells [16]. To overcome this limitation, Z-stacking at multiple levels has been adopted in WSIs. However, this approach results in a long scanning time and high file size. Despite these obstacles, WSIs, obtained under quality control programs offer multiple advantages over glass slides, including standardization of the diagnostic material, same high-quality slides, rapid access via web platforms, and no risk related to transport [17]. In the 2020 proficiency test, 107 institutions conducted the test, using WSIs. The results showed that the distribution of discordance and concordance in the institutions selecting WSIs were not different from those in the institutions using glass slides. We look forward to greater adoption and benefits from WSIs in various fields of quality control in the future.
The change of Korean cytopathology practice can be evidently associated with COVID-19 pandemic. However, the possibility of the additional factors that might be associated with those changes, cannot be rejected completely. Thus, further study would be necessary that makes an effort to analyze the complex factors which could cause the change of cytopathology practice during COVID-19 pandemic.
In summary, CQI during the COVID-19 pandemic was characterized by a reduction in the number of cytologic examinations. This change was the most prominent for respiratory-related specimens, including sputum and bronchial washing/brushing. LBC coverage has markedly increased due to the reduction of conventional sputum smears. Other indicators of quality assurance, including the distribution of GYN sample cytologic diagnoses, gynecologic cytology-histology correlation review, adequacy of GYN samples, proficiency test results, and sample adequacy assessment were shown to be well maintained, despite the social and medical crises from the COVID-19 pandemic.
Supplementary Information
The Data Supplement is available with this article at https://doi.org/10.4132/jptm.2022.09.21.
Notes
Ethics Statement
This study was reviewed and approved by the Institutional Review Board of The Catholic University of Korea, Uijeongbu St. Mary’s Hospital (UC21ZCSI0062) and received a waiver of the need to obtain informed patient consent.
Availability of Data and Material
The datasets generated or analyzed during the study are available from the corresponding author on reasonable request.
Code Availability
Not applicable.
Author contributions
Conceptualization: YC, SAH, GG. Data curation: YC, SAH. Investigation: YC, SAH. Methodology: YC, SAH. Project administration: YC, SAH. Resources: YC, SAH. Supervision: GG. Validation: YC, SAH. Visualization: YC, SAH, HJ, SSK, MSJ, JSP, JYJ, YC. Writing—original draft: YC, SAH, HJ, SSK, MSJ, JSP, JYJ, YC. Writing—review & editing: YC, SAH, HJ, SSK, MSJ, JSP, JYJ, YC, GG.
Conflicts of Interest
YC, a contributing editor of the Journal of Pathology and Translational Medicine, was not involved in the editorial evaluation or decision to publish this article. All remaining authors have declared no conflicts of interest.
Funding Statement
No funding to declare.