Reevaluating diagnostic categories and associated malignancy risks in thyroid core needle biopsy
Article information
Abstract
As the application of core needle biopsy (CNB) in evaluating thyroid nodules rises in clinical practice, the 2023 Korean Thyroid Association Management Guidelines for Patients with Thyroid Nodules have officially recognized its value for the first time. CNB procures tissue samples preserving both histologic structure and cytologic detail, thereby supplying substantial material for an accurate diagnosis and reducing the necessity for repeated biopsies or subsequent surgical interventions. The current review introduces the risk of malignancy within distinct diagnostic categories, emphasizing the implications of noninvasive follicular thyroid neoplasm with papillary-like nuclear features on these malignancy risks. Prior research has indicated diagnostic challenges associated with follicular-patterned lesions, resulting in notable variation within indeterminate diagnostic categories. The utilization of mutation-specific immunostaining in CNB enhances the accuracy of lesion classification. This review underlines the essential role of a multidisciplinary approach in diagnosing follicular-patterned lesions and the potential of mutation-specific immunostaining to strengthen diagnostic consensus and inform patient management decisions.
Core needle biopsy (CNB) has been established as an alternative to fine needle aspiration (FNA) cytology in assessing thyroid nodules, particularly in cases where initial FNA results are nondiagnostic or indeterminate [1,2]. Recent studies advocate CNB as a primary diagnostic tool [1-7]. CNB of the thyroid yields tissue samples that maintain histologic structure and cytologic detail. Moreover, CNB provides substantial tissue for histologic examination and supplementary ancillary testing [8,9]. CNB allows for an exact diagnosis and reduces the need for repeated biopsies or subsequent surgical interventions in cases such as lymphoma, medullary thyroid carcinoma, anaplastic thyroid carcinoma, other rare thyroid diseases, and diseases of non-thyroid origin [1,9].
With CNB becoming increasingly prevalent in clinical practice, issues have been raised about the appropriate use of diagnostic categories and the corresponding risk of malignancy (ROM). In Korea, the inaugural pathology reporting system for thyroid CNB was introduced in 2015 and later revised in 2019 by the Clinical Practice Guidelines Development Committee of the Korean Thyroid Association [9,10]. Despite the Korean Endocrine Pathologists reaching a consensus on the pathology report of thyroid CNB [9,10], the implied ROM for each diagnostic category has yet to be quantified. In the course of calculating the malignancy risk estimates within the diagnostic categories, various factors warrant consideration. These encompass patient demographics, criteria for nodule selection, differences in pathologist expertise and the application of diagnostic criteria, possible overestimation of malignancy risk for some diagnostic categories based exclusively on post-surgical thyroid cases, publication bias, and the surgically proven histological diagnosis of noninvasive follicular thyroid neoplasm with papillary-like nuclear features (NIFTP). Recently, the 2023 Korean Thyroid Association Management Guidelines for Patients with Thyroid Nodules made a significant stride by addressing the implied ROM for each diagnostic category for the first time [11]. Accordingly, this article presents a concise review that illuminates these recent updates concerning the ROM within thyroid CNB categories.
FORMAT OF THYROID CORE NEEDLE BIOPSY REPORTS
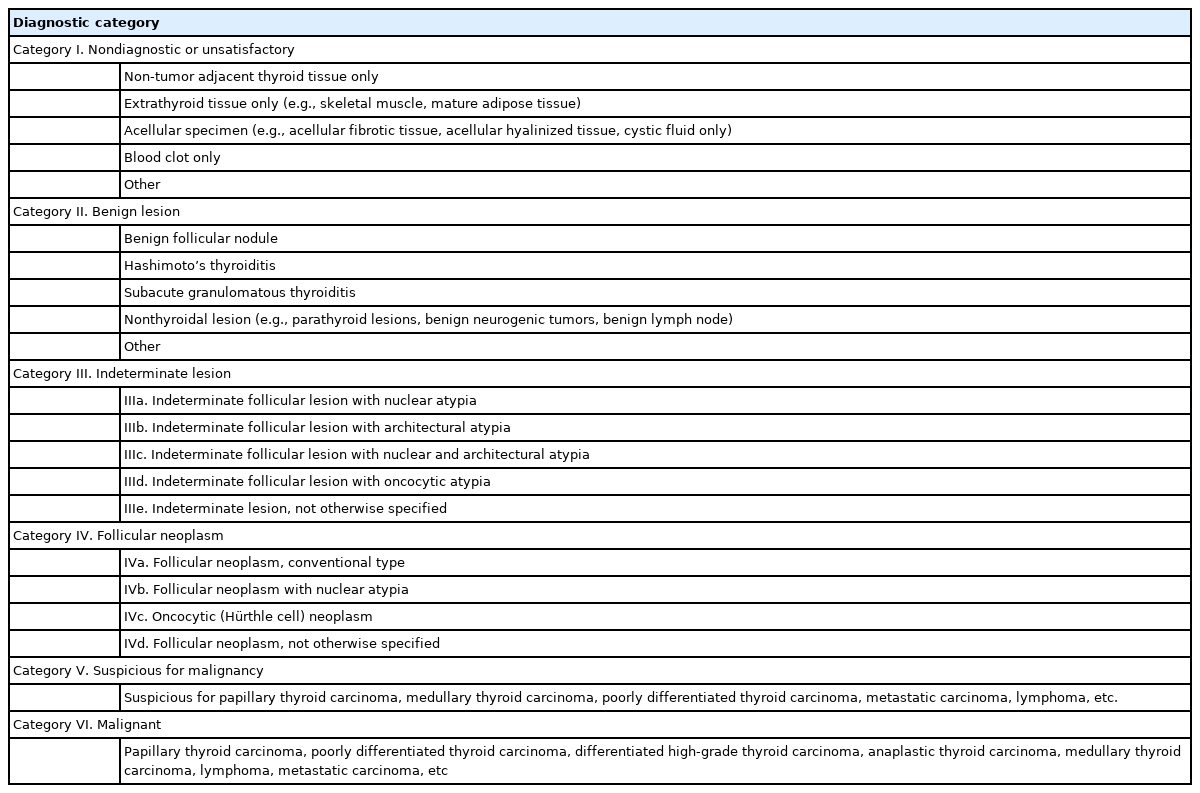
Thyroid CNB reports adhere to a structured format consisting of a diagnostic category, a subcategory, and a microscopic description (Table 1) [9]. The six diagnostic categories include (1) nondiagnostic (category I); (2) benign lesion (category II); (3) indeterminate lesion (category III); (4) follicular neoplasm (category IV); (5) suspicious for malignancy (category V); and (6) malignant (category VI). Categories III and IV are further divided into subcategories based on the status of nuclear atypia, architectural atypia, or oncocytic atypia. Subcategories that exhibit nuclear atypia (categories IIIa, IIIc, and IVb) raise concerns for conditions such as papillary thyroid carcinoma and NIFTP, which are typically associated with a higher ROM compared to subcategories that display architectural or oncocytic atypia [8,12-16].
RISK OF MALIGNANCY OF THYROID CORE NEEDLE BIOPSY DIAGNOSTIC CATEGORIES
Based on retrospective studies in the literature, the frequency and ROM for each category are summarized in Table 2. The ROM estimates for each category are based on clinical follow-up and surgically resected nodules. Benign nodules within category II were clinically confirmed based on additional benign results from FNA or CNB and stable or reduced nodule size observed over a 1-year ultrasound follow-up period. Pathological confirmations on surgical specimens were used for nodules within categories I, III, IV, V, and VI.

Diagnostic frequency and implied risk of malignancy according to the diagnostic category of thyroid CNB
The method of estimating the cancer risk, which is based on histologic follow-up, overestimates the ROM, particularly for the categories I–III, where there is selection bias given the relatively small proportion of nodules that undergo excision. Although NIFTP is a surgical disease and cannot be preoperatively diagnosed on CNB or FNA specimens, the morphologic features of NIFTP tend to lead to classification on CNB/FNA as either category III, IV, or V, thereby impacting the resultant ROM calculations [13,14,17,18]. The ROM for each category is shown when including and excluding NIFTP in malignancy, information that might help guide more conservative clinical management of some nodules. The presence of NIFTP lowers the ROM within the diagnostic category of the thyroid CNB reporting system. The corresponding modifications in the ROM within categories III and IV have been deduced from the observed shifts in malignancy risk from three retrospective studies [13,14,17].
DIAGNOSTIC CHALLENGES IN FOLLICULAR-PATTERNED LESIONS ON CORE NEEDLE BIOPSY
CNB has emerged as a viable alternative to FNA, serving to decrease inconclusive results in the diagnosis of thyroid nodules. Nonetheless, follicular-patterned lesions presented in CNB continue to pose a significant challenge to pathologists, often falling within diagnostic categories II, III, or IV. The diagnostic rates of CNB categories may fluctuate based on pathologists’ diagnostic thresholds for follicular-patterned lesions [13,14,17].
The histologic architecture and the status of the tumor capsule are critical determinants in diagnosing follicular-patterned lesions. Within the context of CNB specimens, follicular proliferative lesions characterized by a discernible tumor capsule are typically classified as category IV (follicular neoplasm) [9,10]. Conversely, in specimens where a tumor capsule is not apparent, these lesions are generally classified as category II (benign) in the absence of nuclear or architectural atypia, or category III (indeterminate follicular lesion) when nuclear or architectural atypia is present.
In addition to the condition of the tumor capsule, the histologic architecture also plays a significant role in determining the diagnosis of follicular-patterned lesions. A microfollicular proliferative lesion, distinctly separated from the surrounding normal parenchyma by a fibrous capsule, leans towards a diagnosis of a category IV (follicular neoplasm). In contrast, a CNB specimen exhibiting a primarily microfollicular or trabecular growth pattern, but lacking a discernible fibrous capsule or adjacent nonlesional tissue, typically falls into category IIIb (indeterminate follicular lesion with architectural atypia). This categorization stems from the uncertainty regarding the presence of a tumor capsule in the lesion. This subcategory exhibits no nuclear atypia. However, certain pathologists might classify such cases under category IV, particularly when ultrasound imaging reveals a solitary nodule with a peripheral hypoechoic halo indicative of a tumor capsule. It is noteworthy that, even if a follicular-patterned lesion presents a definitive fibrous capsule, if the lesion exhibits a macrofollicular pattern rather than microfollicular or trabecular patterns, some pathologists might lean towards categorizing it as category III [15,16]. This preference is motivated by the understanding that a macrofollicular pattern is characteristic of benign thyroid diseases, even when a definitive fibrous capsule associated with a macrofollicular proliferative lesion is present.
A comparison of the diagnostic categories from three major Korean hospitals, all of which perform a high volume of thyroid CNBs, reveals a noticeable variation. One hospital reported the frequencies of categories II, III, and IV as 38.1%, 17.6%, and 10.2%, respectively [14]. Another hospital reported the frequencies as 38.3%, 24.5%, and 7.6%, respectively [17]. Contrastingly, at our institution, the frequencies were markedly different at 60.9%, 1.2%, and 17.5%, respectively [13]. It is noteworthy that the occurrence of category III was significantly lower in our hospital than in the others, while the incidences of categories II and IV were comparatively higher in our hospital than the others.
CNB tends to diagnose category IV “follicular neoplasms” more frequently than FNA [13,17,19], leading to an increased number of patients undergoing surgery based on CNB diagnoses compared to FNA. While the rate of follicular neoplasms diagnosed using CNB may differ significantly across various institutions, potentially due to the variability in how different observers interpret architectural or nuclear atypia in CNB, the ROMs found post-surgery for these follicular neoplasms remain consistent [13,14,17]. This consistency is observed irrespective of whether the initial diagnosis was made via CNB or FNA [13].
CLASSIFICATION OF FOLLICULAR-PATTERNED LESIONS USING MUTATION-SPECIFIC IMMUNOSTAINING IN CORE NEEDLE BIOPSY
Immunostaining has served as an essential tool, supplementing standard methodologies in clarifying differential diagnoses in surgical pathology. This straightforward and economical technique facilitates the identification of lineage or cell type in histopathology and cytopathology. The recent emergence of innovative markers, particularly mutation-specific markers and those bearing translational significance, has substantially transformed the paradigm of immunohistochemistry (IHC). These progressive strides have notably influenced clinical practice and investigative pursuits within the field of thyroid disease.
The RAS genetic variants, typically found to be mutually exclusive with BRAF variants, are the most common oncogenic changes observed in follicular-patterned thyroid tumors [5,34,35]. Of all RAS variants identified in thyroid tumors, RAS Q61R is the most prevalent [8,36]. IHC for RAS Q61R, using the SP174 antibody, has proven to be an accurate method for detecting thyroid tumors harboring the RAS Q61R variant [8,37]. While RAS variants are not inherently sensitive or specific markers for thyroid cancers, their detection in preoperative FNA or CNB samples exhibiting indeterminate results usually prompts a diagnostic lobectomy. Thus, for CNB samples wherein histologic morphologies complicate the differential diagnosis between categories III and IV, a positive result for RAS Q61R IHC often simplifies the diagnostic process, favoring a categorization into category IV. In cases where a CNB specimen primarily displays a microfollicular or trabecular growth pattern without a discernible fibrous capsule, it is typically diagnosed as an indeterminate follicular lesion (category III). However, a positive RAS Q61R result in such a case could lead to a revised diagnosis of a follicular neoplasm (category IV) (Figs. 1, 2).

Core needle biopsy of a microfollicular proliferative lesion exhibiting morphological differences from adjacent thyroid tissue. (A) Although the lesion is distinctly segregated from surrounding tissue, the absence of a discernible tumor capsule typically leads to its categorization under category III based on histomorphology. (B) Immunohistochemistry for RAS Q61R clearly delineates immunostaining-positive tumor cells from the immunostaining-negative normal thyroid tissue. Ultimately, with the incorporation of immunostaining results, the specimen should be appropriately diagnosed as follicular neoplasm, conventional type (category IVa).

The core needle biopsy displays a microfollicular proliferative lesion that exhibits morphological differences from the adjacent thyroid tissue, but lacks a fibrous capsule (A). (B) The tumor component tests positive for RAS Q61R immunostaining. (C) A high-power view reveals microfollicles lined by tumor cells exhibiting nuclear atypia and thin fibrous bands within the stroma. (D) Tumor cells show cytoplasmic and membranous positivity for RAS Q61R. This specimen should be appropriately classified as a follicular neoplasm with nuclear atypia (category IVb). After conducting a diagnostic lobectomy, the definitive pathological diagnosis was confirmed as an invasive encapsulated follicular variant of papillary thyroid carcinoma.
When CNB samples display nuclear atypia yet lack sufficient histologic features for a definitive malignancy diagnosis, they may be assigned to category IIIa (Indeterminate follicular lesion with nuclear atypia) or category V (suspicious for malignancy) based on the extent of nuclear atypia and the quantity of atypical cells involved. In such situations, employing BRAF VE1 IHC to detect the BRAF p.V600E variant can be valuable for differential diagnosis (Fig. 3). Given the demonstrated reliability of BRAF VE1 IHC in identifying the BRAF p.V600E across different tumor types, including thyroid cancers [34,38], a positive result from this method has the potential to clarify indeterminate CNB results, typically pointing towards a definitive diagnosis of papillary thyroid carcinoma.

The core needle biopsy specimen exhibits both follicular and abortive papillary architecture. (A) In light of the nuclear atypia and predominantly follicular growth, the diagnostic considerations span from category III to IV. (B) The positive result for BRAF VE1 immunostaining confirmed the diagnosis, categorizing the specimen as category VI - papillary thyroid carcinoma. The inset image provides a magnified view of the indicated region as a square, facilitating the observation of nuclear atypia. Arrows indicate abortive papillae.
RISK STRATIFICATION OF PREOPERATIVELY DIAGNOSED INDETERMINATE FOLLICULAR-PATTERNED LESIONS
Recently, our team established that CNB categories III and IV could be classified into two distinct risk groups based on histologic features of nuclear atypia and IHC for RAS Q61R [8]. CNBs exhibiting nuclear atypia or RAS Q61R expression were identified as high-risk, bearing an average NIFTP/malignancy risk of 75.5%. In contrast, CNBs without these findings were marked as low-risk, having a risk of approximately 34.9%. RAS Q61R IHC has shown high sensitivity and specificity for identifying the RAS p.Q61R variant and demonstrates a positive predictive value of 74.3% and a negative predictive value of 55.5% for diagnosing NIFTP/malignancy in CNB categories III and IV. Follicular lesions exhibiting both nuclear atypia and RAS Q61R expression carry an 86% NIFTP/malignancy risk. Therefore, in patients classified as CNB category III/IV, the presence of nuclear atypia or RAS Q61R expression signifies a heightened risk of NIFTP/malignancy, which may necessitate consideration for surgical resection (Figs. 4, 5). On the other hand, thyroid nodules lacking nuclear atypia and RAS Q61R expression, which present a lower likelihood of NIFTP/malignancy, might be suitable for observation without further intervention. This approach can potentially assist in the management of patients presenting with indeterminate CNB results.

The core needle biopsy specimen reveals a microfollicular proliferative lesion surrounded by a thick fibrous capsule (A) and exhibiting positivity for RAS Q61R immunostaining (B). Considering these characteristics, the specimen can be confidently classified as follicular neoplasm, conventional type (category IVa). After a diagnostic lobectomy, the tumor was conclusively identified as follicular thyroid carcinoma.

The core needle biopsy specimen reveals a follicular proliferative lesion characterized by follicles of varying sizes and a discernible fibrous capsule (A), and demonstrates positivity for RAS Q61R immunostaining (B). (C) A high-power view reveals follicles lined by tumor cells exhibiting nuclear atypia, intermixed with follicles comprised of cells with no nuclear atypia. (D) Tumor cells show cytoplasmic and membranous positivity for RAS Q61R. The specimen was diagnosed as a follicular neoplasm with nuclear atypia (category IVb). Following a diagnostic lobectomy, the definitive pathological diagnosis was established as noninvasive follicular thyroid neoplasm with papillary-like nuclear features.
In patients preoperatively diagnosed with follicular neoplasm (category IV), nodule size plays a critical role in surgical decisionmaking due to the rising ROM associated with increasing nodule size. The 2023 Korean Thyroid Association Management Guidelines for Patients with Thyroid Nodules states that surgical intervention is typically favored for follicular neoplasms of 2 cm or more, given the escalating malignancy risk [11]. Even for tumors smaller than 2 cm, a malignancy risk exists, necessitating consideration of diagnostic surgery based on individual clinical findings [11]. A meta-analysis of 14 observational studies involving 2,016 thyroid nodules diagnosed as follicular neoplasms (category IV) demonstrated that in comparison to their smaller counterparts, nodules measuring 4 cm or larger, 3 cm or larger, and 2 cm or larger were associated with a respective 2.29-fold (95% confidence interval [CI], 1.68 to 3.11), 2.39-fold (95% CI, 1.45 to 3.95), and 1.63-fold (95% CI, 1.13 to 2.35) increase in ROM for thyroid nodules of various sizes [39-52].
Molecular marker tests can facilitate malignancy assessment and inform decisions regarding ultrasound monitoring or surgical interventions. These decisions should account for patient preferences, clinical feasibility, and evaluations supplemented by ultrasound findings [20]. While next-generation sequencing (NGS)- based molecular panel testing is predominantly employed in Western countries, such molecular NGS testing for diagnostic or prognostic prediction on preoperative biopsy specimens is not currently permitted in Korea [11].
CONCLUSION
The use of CNB for the evaluation of thyroid nodules has seen an increasing trend due to its potential as an alternative to FNA cytology. The 2023 Korean Thyroid Association Management Guidelines for Patients with Thyroid Nodules have recognized the value of CNB for the first time. Despite its advantages, the diagnosis of follicular-patterned lesions remains subject to interobserver variability. Therefore, a multidisciplinary approach emphasizing clinicopathologic correlation is essential. Furthermore, the utilization of mutation-specific immunostaining can enhance diagnostic consensus and contribute meaningfully to patient management by informing clinical decision-making processes.
Notes
Ethics Statement
Not applicable.
Availability of Data and Material
Data sharing not applicable to this article as no datasets were generated or analyzed during the study.
Code Availability
Not applicable.
Conflicts of Interest
C.K.J., the editor-in-chief of the Journal of Pathology and Translational Medicine, was not involved in the editorial evaluation or decision to publish this article.
Funding Statement
This research was supported by a grant (HI21C0940) from the Korean Health Technology R&D Project, Ministry of Health and Welfare, Republic of Korea.