Exploring histological predictive biomarkers for immune checkpoint inhibitor therapy response in non–small cell lung cancer
Article information
Abstract
Treatment challenges persist in advanced lung cancer despite the development of therapies beyond the traditional platinum-based chemotherapy. The early 2000s marked a shift to tyrosine kinase inhibitors targeting epidermal growth factor receptor, ushering in personalized genetic-based treatment. A further significant advance was the development of immune checkpoint inhibitors (ICIs), especially for non–small cell lung cancer. These target programmed death-ligand 1 (PD-L1) and cytotoxic T lymphocyte antigen 4, which enhanced the immune response against tumor cells. However, not all patients respond, and immune-related toxicities arise. This review emphasizes identifying biomarkers for ICI response prediction. While PD-L1 is a widely used, validated biomarker, its predictive accuracy is imperfect. Investigating tumor-infiltrating lymphocytes, tertiary lymphoid structure, and emerging biomarkers such as high endothelial venule, Human leukocyte antigen class I, T-cell immunoreceptors with Ig and ITIM domains, and lymphocyte activation gene-3 counts is promising. Understanding and exploring additional predictive biomarkers for ICI response are crucial for enhancing patient stratification and overall care in lung cancer treatment.
Lung cancer is characterized by a high proportion of cases diagnosed as unresectable, locally advanced or metastatic disease, and among various prevalent cancers, it stands out as one with a poor prognosis even at the time of diagnosis [1,2]. Advances have revolutionized lung cancer treatment, with platinum-based chemotherapy serving as a longstanding cornerstone. With the development of tyrosine kinase inhibitors targeting epidermal growth factor receptor (EGFR) in the early 2000s, targeted therapy has significantly improved the survival of patients with lung cancer [3]. Subsequent to EGFR, a deeper understanding of the molecular genetics of lung cancers, including ALK, ROS-1, and KRAS, has led to the identification of new molecular targets for personalized treatment based on individual carcinogenic genetic characteristics. However, more than half of lung cancer patients do not benefit from targeted therapies, which poses a major limitation to their application [3].
The third major revolution in lung cancer treatment was brought by the emergence of immunotherapy in the form of immune checkpoint inhibitors (ICIs) [4-6]. The discovery of immune checkpoints and the subsequent development of Nobel Prize-winning ICIs have facilitated revolutionary changes in lung cancer treatment, particularly in non–small cell lung cancer (NSCLC) [7]. ICIs targeting key immune evasion genes, such as programmed death-ligand 1 (PD-L1) and cytotoxic T lymphocyte antigen 4 (CTLA-4), have been developed based on the immunological understanding of the interaction between tumor cells and immune cells. These inhibitors have been used as a part of first-line therapy for various cancers [5,6]. Inhibition of PD-L1 and CTLA-4–related pathways primes cytotoxic T cells and induces anti-tumor activity [6]. ICIs have become an integral part of their clinical management in NSCLC patients without driver mutations [8].
Despite the growing use of ICIs, some patients are missing out on the chance to benefit from them. Moreover, immune-related toxicities can adversely impact the quality of life for some patients or even cause mortality [9,10]. With the widespread application of ICIs in treating patients with NSCLC, the appropriate selection of patients likely to benefit from this therapy has become increasingly important. Hence, it is imperative to identify clinically useful biomarkers for this purpose. PD-L1 expression, along with tumor mutational burden and mismatch gene repair deficiency, has been validated as a predictive marker for ICI response and has received U.S. Food and Drug Administration (FDA) approval for companion diagnostics [11]. Other markers, such as tumor mutational burden and microsatellite instability, have also been recognized as predictive markers of ICI response [11-13]. However, the search for a perfect and reliable biomarker for ICI response is ongoing, and advanced molecular techniques are not readily available in many clinical situations. In this narrative review, we focus on histological predictive markers observable during the pathological diagnostic process or through immunohistochemical staining to provide valuable insights into the nuanced world of predictive biomarkers beyond PD-L1 (Fig. 1).
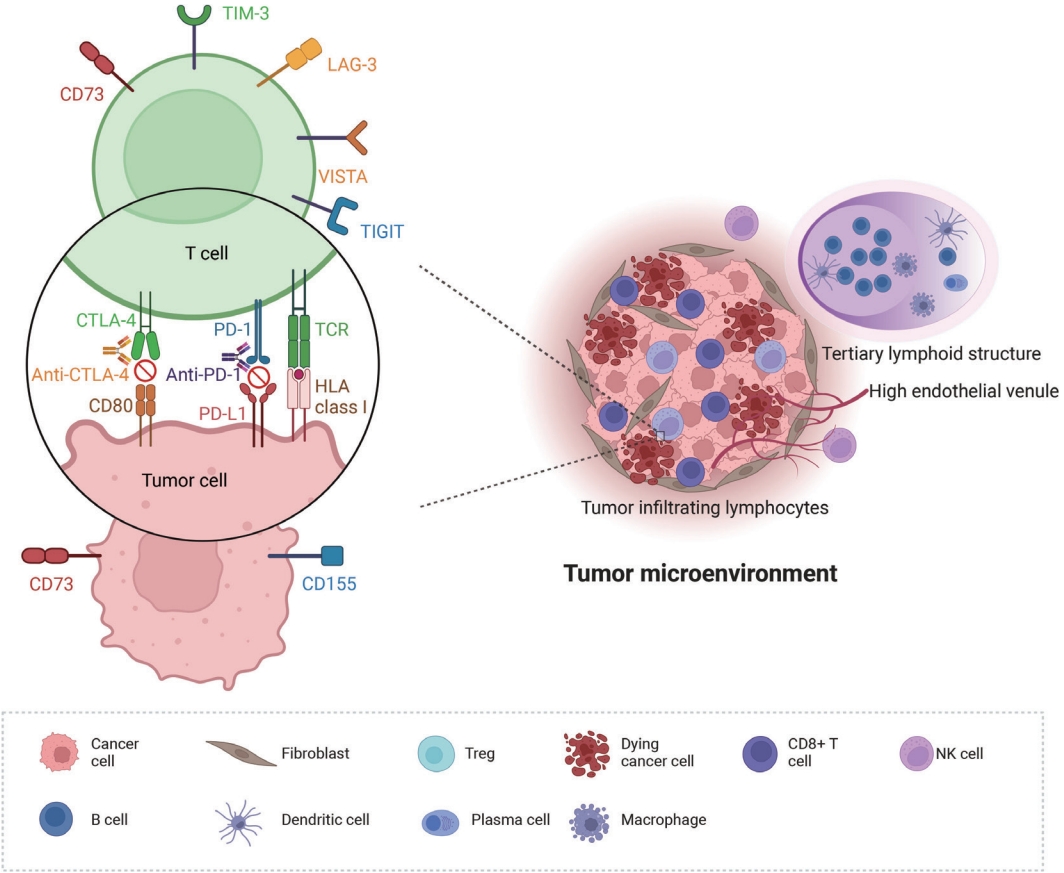
Predictive biomarkers for immune checkpoint inhibitor therapy in non–small cell lung cancer that could be observed through histological or immunohistochemical observations. CTLA-4, cytotoxic T lymphocyte antigen 4; HLA, human leukocyte antigen; LAG-3, lymphocyte activation gene-3; NK, natural killer; PD-1, programmed death-1; PD-L1, programmed death-ligand 1; TCR, T-cell receptor; TIGIT, T-cell immunoreceptors with Ig and ITIM domains; TIM-3, T cell immunoglobulin and mucin domain-3; Treg, regulatory T cells; VISTA, V-domain immunoglobulin suppressor of T cell activation.
BIOMARKERS OF RESPONSE AND RESISTANCE TO IMMUNOTHERAPY IN NON–SMALL CELL LUNG CANCER
PD-L1 expression
PD-L1 expression is currently the only recommended biomarker in the National Comprehensive Cancer Network guidelines for determining the treatment approach to metastatic NSCLC, excluding genomic driver mutations [8]. It is also the sole companion diagnostic test for PD-L1 inhibitors (Fig. 2A) [12,14]. PD-L1 expression differs in different subtypes of NSCLC. In a literature review of 42 studies, PD-L1 expression was higher in squamous cell carcinoma than in adenocarcinoma, 41.05% vs. 34.72% at >1% cutoff [15]. Other subtypes of NSCLC have a limited number of case studies. However, sarcomatoid carcinoma, an aggressive, poorly differentiated subtype of NSCLC, demonstrated an impressively high rate of PD-L1 positivity. In a cohort of 41 patients, 78% of the patients showed positivity at PD-L1 SP142 ts, and a study by Domblides et al. [16] had 94.7% positivity at PD-L1 SP263 >5% [17]. Several clinical trials have demonstrated the predictive capability of PD-L1 expression in stage IV NSCLC [4]. These trial results both suggest that PD-L1 expression assists in patient selection and indicate its potential to predict the extent of a patient’s treatment response to PD-L1 inhibitors [12]. However, certain clinical trials have disputed the utility of programmed death-1 (PD-1)/PD-L1 expression in predicting treatment outcomes in patients receiving these inhibitors [18].
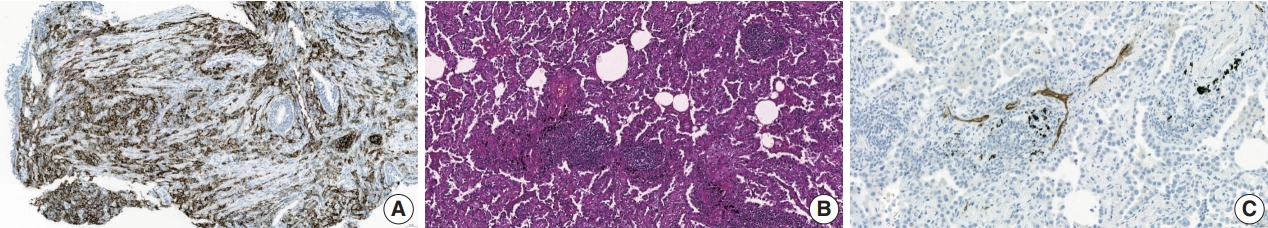
(A) Lung adenocarcinoma tumor cells are expressing programmed death-ligand 1 (PD-L1). PD-L1 is the most validated predictive biomarker of immune checkpoint inhibitors for patients with lung non-small cell carcinoma (PD-L1 clone SP263). (B) Lymphoid aggregate within lung adenocarcinoma forms a tertiary lymphoid structure. (C) The high endothelial venules are identified through positive staining with MECA-79 antibody (MECA-79 staining).
PD-L1 expression does not provide absolute certainty in predicting response or resistance to treatment. Some studies of a PD-L1 <1% expression subgroup highlighted the need for novel and complementary methods to identify patients who respond to ICIs [18,19]. In the multicohort, open-label phase 1 CheckMate 012 trial, increasing PD-L1 expression level was associated with greater benefit, with an overall response rate of 50%, in which nivolumab was used as a first-line therapy for advanced NSCLC; however, clinical activity was also observed in a patient population with <1% PD-L1 expression [20]. The PD-L1 inhibitor pembrolizumab was administered to patients with a ≥50 PD-L1 tumor proportional score or combined positive score [21] (Table 1). Although PD-L1 is widely used as a biomarker in clinical practice, and PD-L1 positivity generally predicts therapeutic response, its predictive value is not absolute. Challenges associated with using PD-L1 as a biomarker include inter- and intra-tumor heterogeneity of PD-L1 expression, diversity in PDL1 assay methods, cutoff values, and interobserver bias [21,22]. To overcome one of the challenges, an artificial intelligence-powered PD-L1 analyzer was applied to PD-L1 scoring of NSCLC slides, and it was shown to improve pathologists’ consensus scoring and prediction of therapeutic response [23]. Such an effort would increase the reliability of PD-L1 interpretation in the future.
Tumor-infiltrating lymphocytes
Tumor-infiltrating lymphocytes (TILs) have undergone extensive study, revealing their crucial role in mediating the immune system’s anti-tumor activity. Additionally, tumor-immune system interactions have revealed insights into the causes of immunotherapy resistance [5]. Analyzing TIL nature at diagnosis both predicts immunotherapy outcomes and informs treatment strategies. Traditionally, cancer research centered on malignant cells, neglecting tumor microenvironment components. However, it has gradually been recognized that tumor cells possess antigenic properties, inducing an immune response of producing altered proteins that the host immune system perceives as harmful [24].
Solid tumors encompass various cellular components in the tumor microenvironment, including the extracellular matrix, stromal, endothelial, and immune cells [25]. The density, location, and organization of immune cells collectively constitute the “immune context” [26]. The immune context at diagnosis correlates with clinical outcomes in various tumors, such as melanoma and colorectal, lung, and breast cancers [26]. The prognostic value of immune cells is based on their functional status and distinct phenotypes. Cytotoxic T cells, helper T cells, natural killer cells, and dendritic cells contribute to anti-tumor responses. In contrast, FOXP3-positive regulatory T cells (Tregs) and myeloid-derived suppressor cells have pro-tumorigenic effects and promote cancer growth and invasion [27]. Each immune cell type has different effects, so they carry distinct prognostic significance [26]. The prognostic importance of TIL in breast cancer has been extensively studied [28]. The association between TIL and breast cancer prognosis was first investigated by Aaltomaa et al. in the early 1990s [29]. Since then, several researchers have reported the prognostic and predictive value of TIL in breast cancer, and these results have been consistent across various randomized clinical trials [30]. Several studies have evaluated TIL as a continuous parameter in hematoxylin and eosin– stained tumor sections following the criteria proposed by Denkert et al. [31]. International collaborative efforts have sought to standardize TIL assessment and enhance reproducibility, and recommend scoring stromal TILs as a percentage of stromal areas between nests of carcinoma cells while excluding the areas occupied by the carcinoma cells from the total assessed surface area [32]. TIL assessment guidelines established for breast cancer are also being applied to NSCLC, melanoma, gastrointestinal tract carcinomas, and other solid tumors [33,33].
In solid tumors, TILs primarily consist of CD4+ and CD8+ T cells [34]. CD8+ T cells act as cytotoxic T cells and can directly kill tumor cells. Increased CD8+ T-cell infiltration has been associated with improved patient survival [35,36]. CD4+ T cells function as helper T cells or Tregs that mediate diverse functions through cytokine secretion. Th cells recruit leukocytes, stimulate phagocytes and cytotoxic T cells to kill tumor cells, and promote B-cell antibody production [37]. In contrast, CD4+ FOXP3+ CD25high Treg cells, which express the transcription factor FOXP3, inhibit effector T cells by secreting cytokines such as transforming growth factor β and interleukin-10 or metabolites like adenosine, thereby conferring immunotolerance. Consistently, the presence of CD4+ FOXP3+ CD25high Tregs has been associated with worsened prognosis in breast cancer and is a marker of poor prognosis in NSCLC [38]. The proportion and type of TILs, along with their organizational level, could make them crucial biomarkers for improving candidate selection for ICI therapy. Although the distribution of CD markers (which reflect immune profiles in the tumor microenvironment) is a potential predictive biomarker of ICI effectiveness in NSCLC, comprehensive analyses or randomized clinical trials are yet to conclusively establish the utility of TIL as definitive predictive biomarkers [8,39,40]. Attempts have been made to create models that combine PD-L1 expression and TIL infiltration to classify the tumor microenvironment and discriminate tumors that respond best to PD-1 inhibitors [41]. Combining multiple biomarkers may be a rational approach for tailoring immunotherapeutic treatments and should be integrated into future clinical trials [42,43].
Theoretically, TILs serve as the main activators of anti-tumor immunity, and if objectively measured in the entire tumor microenvironment, they could be a promising biomarker. However, quantifying TILs is labor-intensive and limited by spatial distribution in whole-slide images and interobserver heterogeneity [44]. Therefore, establishing clinically relevant TIL cutoff values is challenging. The current immunophenotyping concept is based on TIL status in the tumor microenvironment, dividing it into inflamed (intratumorally distributed TILs), immune-excluded (TILs excluded from the cancer stroma), and immune-desert (scant TILs in the tumor microenvironment) subtypes [45]. Although pathologists can easily confirm the abundance of TIL through microscopic examination, TIL quantification is challenging. Some cancer types lack lymphocytes, while others, such as lymphoepitheliomas, show tumor cells engulfed by lymphocytes. Although studies have suggested that immune phenotype predicts clinical outcomes of ICI therapy [11], there is a lack of standardized methodology for quantifying TILs. To address these issues, artificial intelligence–assisted methods have been introduced [46], and ongoing research studies are exploring TIL quantification using multiplex immunohistochemistry and spatial studies [47].
Tertiary lymphoid structures
Tertiary lymphoid structures (TLSs) are ectopic lymphoid formations that develop under prolonged inflammatory conditions, including cancer. Compared to B, T, and dendritic cells, TLSs exhibit varying levels of organization, ranging from locally concentrated immune cell aggregates to mature follicles with well-defined B-cell follicles and germinal centers (Fig. 2B) [48]. Immature TLSs display visible immune cell foci with segregated B and T cell zones but lack follicular dendritic cells and germinal centers, which are crucial sites for B-cell proliferation and affinity maturation [6]. TLS status demonstrates prognostic potential in various cancer types, including NSCLC, colon cancer, breast cancer, and melanoma [48,49]. Additionally, TLS status appears to have a predictive value in ICI therapy; it is strongly associated with improved survival and clinical outcomes in patients receiving ICI treatment for solid tumors such as sarcoma, melanoma, and renal cell carcinoma [50,51]. Recently, Helmink et al. [52] studied the association between renal cell carcinoma and TLS status via spatial transcriptomic analysis of formalinf-ixed paraffin-embedded samples and reported higher remission rates and longer progression-free survival in patients with TLSpositive tumors treated with ICI than in those with TLS-negative tumors.
Some researchers argue that both the presence of immune cells and their organization into TLSs are crucial in response to immunotherapy [53]. However, the reason for the increase in TLS density in ICI responders during treatment remains unclear. Nonetheless, histologically evaluated CD20 density is higher at baseline in responding patients than in non-responding patients, with a further increase observed after ICI treatment [52]. Nevertheless, changes in TLSs due to ICI therapy require prospective validation in larger and more homogeneous patient cohorts for conclusive evidence, which could establish TLSs as an active and beneficial component of ICI treatment.
The exact contribution of TLSs to anti-tumor responses has yet to be fully understood. B cells, a component of TILs, primarily reside within TLSs. The role of B cells in anti-tumor immunity is controversial, with studies suggesting that they contribute to humoral anti-tumor immune responses by generating antibodies against tumor-associated antigens and enhancing cellular immunity by secreting cytokines that increase the activation of antigen-presenting cells (APCs) [54,55]. Theoretically, intra-tumoral TLSs may lead to a complete B-cell response within the tumor, causing a direct anti-tumor effect by maintaining B-cell maturation and antibody production. Notably, B cells may alter T-cell activation and function, contributing to the enhanced therapeutic effects of ICI [54].
These findings could have clinical applications in improving patient selection for ICI therapy, as TLSs can be easily detected in standard pathology laboratories. Prospective studies employing TLSs to select ICI candidates were reported in 2022. In one study, patients with advanced soft-tissue sarcoma known to have limited responses to ICIs were selected for anti–PD-1 therapy based on the presence of TLSs in tumor biopsy specimens. This TLS-positive cohort exhibited improved overall response rates and median progression-free survival compared with the previously unselected cohort. Following this, TLS status has been employed as an inclusion criterion in several ICI clinical trials; more results from similar clinical trials are expected in the near future (NCT04705818 and NCT03475953).
It is noteworthy that previous studies have quantified TLS differently. Some lung cancer studies evaluated TLSs exclusively using the CD208+ dendritic cells present in them. In contrast, others used the follicular dendritic cell markers CD21 and CD23 or assessed TLSs based on the co-localization of CD3+ T cells and CD20+ B cells [48,56]. Although there is currently no consensus on a standardized TLS evaluation method, if clinical trial results with TLS inclusion criteria emerge, the methods used in such studies may be accepted as standardized approaches.
EMERGING PREDICTIVE BIOMARKERS OF IMMUNE CHECKPOINT INHIBITOR THERAPY
High endothelial venule
Tumor-associated high endothelial venules (TA-HEVs) originate from post-capillary venules and are characterized by an elevated expression of high endothelial venule-specific sulfated MECA-79 (PNAd) antigens (Fig. 2C). TA-HEVs play a crucial role in lymphocyte recirculation and TLS formation, and increased HEV values are associated with a favorable prognosis in gastric cancer [57,58]. Recent discoveries suggest that ICIs increase the network of TA-HEVs and enhance CD8+ T-cell infiltration [59]. Furthermore, anti-angiogenic therapy downregulates continuous angiogenic signaling, resulting in vasculature normalization and promoting TA-HEV formation [60,61]. Despite these significant developments, the characterization of TAHEVs in the context of ICI therapy for NSCLC remains poorly defined [62]. Studies have suggested an association between TAHEVs and the tumor microenvironment in solid tumors; however, further investigations are warranted to determine the usefulness of TA-HEVs in selecting patients for ICI therapy [63].
HLA class I
The major histocompatibility complex, the human leukocyte antigen (HLA), includes cell surface molecules responsible for presenting and recognizing self- and non-self peptides. It is encoded by a highly polymorphic gene complex. The HLA gene complex contains more than 200 loci on the short arm of chromosome 6. Population surveys have identified thousands of allelic variants of HLA molecules primarily influenced by the nature and composition of the peptide-binding groove [64], and these variants are associated with the risk of developing various diseases, including cancer [65].
Human leukocyte antigen class I (HLA-I) promotes the clonal amplification and cellular activation of naïve TCD8 lymphocytes by presenting intracellular antigenic peptides. HLA-I exhibits polymorphisms in its antigenic peptide-binding region, allowing each variant to bind to a specific repertoire of peptide ligands [66]. The HLA genotype, which generates this diversity, has been linked to the prognosis of patients receiving ICIs, with certain supertypes associated with improved or lower survival [67]. Beta-2-microglobulin (B2M), a component of HLA-I, is required for antigen presentation by dendritic cells. B2M mutations can lead to resistance to ICI therapy, with B2M abnormalities associated with cancer progression in 29.4% of the cases [68]. B2M mutations or decreased expression have been linked with ICI outcomes in patients with head and neck squamous cell carcinoma and melanoma [69,70].
The HLA class I antigen-derived peptide complex is crucial for presenting tumor antigens to naïve T cells. The activation of naïve T cells requires the interaction of HLA class I-derived peptide complexes with the T cell receptor and co-stimulatory ligands, such as the B7 family, on APCs [64]. The balance between co-stimulatory and co-inhibitory signaling, mediated by immune checkpoint molecules such as PD-1 and CTLA-4, tightly regulates T-cell activation. Some tumor cells evade the host immune system due to defects in their ability to present tumor antigens to naïve T cells via HLA class I molecules [70]. Research is ongoing to investigate the impact of HLA class I expression on the ICI response. In murine solid tumor models, HLA class I expression is reportedly a predictor of ICI response and an overall marker of immunogenicity [71,72]. In vivo studies using HLA class I and class II knockout mice treated with PD-1 antibodies showed strong anticancer effects [73]. Although several in vivo studies have suggested that HLA class I expression may be a predictive marker, clinical evidence remains limited. For instance, in patients with melanoma treated with ICI, post-treatment samples showed significantly lower HLA class I expression, particularly in the progressing metastases of non-responding patients [74]. Retrospective evaluation of patients with metastatic melanoma treated with ipilimumab or nivolumab revealed associations between HLA class I expression and tumor response, with different patterns observed for each treatment [67]. Further studies are underway to analyze whether the patient’s HLA class I subtype influences the ICI response, indicating its potential predictive value.
Novel target immune checkpoint biomarkers
PD-1/PD-L1 and CTLA-4 inhibitors are most widely used for ICI therapy of lung cancer; however, the development of drug resistance remains a challenge. Recently, novel immune checkpoint targets, such as T-cell immunoreceptors with Ig and ITIM domains (TIGIT), have shown promise in preclinical and early clinical studies, offering hope to overcome resistance to conventional ICIs [75]. TIGIT, which is expressed in activated natural killer and regulatory T cells, binds to CD155 (PVR) and CD112 (PVRL2 and nectin-2), ligands on tumor cells and antigen-presenting cells in the tumor microenvironment [75]. A recent randomized phase II clinical trial demonstrated that combining anti-TIGIT antibody tiragolumab and atezolizumab as firstline therapy for advanced PD-L1–positive NSCLC significantly increased objective response rate and progression-free survival compared to the control group. Based on these results, the FDA recently granted breakthrough therapy designation to tiragolumab [75]. TIGIT expression, particularly in CD3+ TIL and peritumoral lymphocyte infiltrates, indicates an “exhausted” T cell phenotype in the tumor microenvironment. Additionally, TIGIT expression positively correlates with PD-1 and PD-L1 density, indicating the synergy between these immune checkpoint axes in lung squamous cell carcinoma and melanoma [76,77]. In lung squamous cell carcinoma tissues analyzed using immunohistochemistry, 85.8% expressed CD155 (PVR) and 26.8% expressed PD-L1. High TIGIT density and high CD155/TIGIT expression correlated with advanced tumor, nodal, and metastasis (TNM) stage and worse overall survival when TIGIT-positive TIL were counted [76]. Although TIGIT expression has been studied in various solid tumors [75-77], data on TIGIT or TIGIT ligands as immunohistochemical biomarkers in NSCLC are limited. Currently, the immunohistochemical status of TIGIT is not a prerequisite for the use of TIGIT inhibitors, and PD-L1 positivity is considered sufficient. No data exist on the potential role of TIGIT as a predictive marker for anti-TIGIT regimens [75]. In most clinical trials, anti-TIGIT agents are administered in combination with anti–PD-1/PD-L1 or anti–CTLA-4 inhibitors; however, some trials on NSCLC have investigated anti-TIGIT monotherapy (NCT02964013, NCT04165070). Further studies are needed to determine the role of TIGIT expression as a predictive marker of response to anti-TIGIT therapy regimens, particularly in lung cancer, which has been the focus of most immunotherapy trials.
Another novel ICI target is lymphocyte activation gene-3 (LAG-3), which is expressed in activated CD4+/CD8+ T cells, regulatory T cells, natural killer (NK) cells, B cells, and plasmacytoid dendritic cells. LAG-3 signaling plays a negative regulatory role in T helper 1 cell activation, proliferation, and cytokine secretion, allowing tumor cells to evade the host immune system [78]. Several LAG-3 inhibitors are under development, and some are undergoing phase II trials as first-line therapies for advanced NSCLC [79]. LAG-3 expression in TIL in NSCLC appears positively correlated with PD-1/PD-L1 expression [80]. However, similar to TIGIT, no prospective study has assessed the potential value of LAG-3 as a predictive NSCLC biomarker.
Other novel ICI targets include T cell immunoglobulin and mucin-domain containing-3, NK group 2 member A (NKG2A), and CD73 [81]. CD73, acting as an immune checkpoint, generates adenosine, inhibiting immune activation via the A2A receptor [82]. CD73 is upregulated in various cancers, including lung cancer, and its overexpression in tumor tissues is associated with poor prognosis [83-85]. In preclinical studies, combination therapy with PD-1/PD-L1 and CD73 inhibitors has demonstrated synergistic anti-tumor effects [86]. CD73 expression in NSCLC positively correlates with a “hot” immune environment, including PD-L1 expression and the presence of TIL [85]. However, more data are necessary to comprehend the effects of CD73 on the tumor microenvironment [87,88]. Retrospective analyses of NSCLC patients treated with anti–PD-1/PD-L1 therapy indicate that high CD73 expression may predict a favorable response, particularly in EGFR-mutant patients [89]. Given the high EGFR mutation rate in the East Asian population, CD73 may be a crucial therapeutic target and predictive marker.
Several other immune checkpoint targets, including V-domain immunoglobulin suppressor of T cell activation (VISTA), B7-H3 (CD276), indoleamine 2,3-dioxygenase 1, glucocorticoid-induced tumor necrosis factor receptor–related receptor, and CD47, are under investigation [81,90]. Ongoing clinical trials are evaluating these inhibitors alone and in combination with PD-1 and PD-L1 inhibitors [90]. As more ICIs are integrated into therapy, discovering predictive markers for optimal patient selection will become increasingly necessary.
CONCLUSION
ICIs are increasingly utilized as standard treatments in clinical settings, particularly for stage III locally advanced NSCLC and extensive-stage small cell lung cancer, with an emphasis on metastatic NSCLC. Future indications for its use are expected to expand. However, additional research is required to identify biomarkers that are more reliable than PD-L1 expression and that are readily usable in daily practice. Discovering a cost-effective, easily accessible predictive biomarker would significantly enhance patient stratification and management, ultimately improving overall patient care. Furthermore, advancements in understanding the mechanisms of resistance to ICIs and strategies to overcome them, along with pathological methods for prediction, are crucial for further progress in this field.
Notes
Ethics Statement
Not applicable.
Availability of Data and Material
Data sharing not applicable to this article as no datasets were generated or analyzed during the study.
Code Availability
Not applicable.
Author Contributions
Conceptualization: UC, HSP. Data curation: UC, SI. Funding acquisition: UC. Investigation: UC. Methodology: UC, SI, HSP. Project administration: UC. Resources: UC, SI, HSP. Supervision: UC. Validation: UC, SI, HSP. Visualization: UC. Writing—original draft: UC. Writing—review & editing: UC, SI, HSP. Approval of the final manuscript: all authors.
Conflicts of Interest
The authors declare that they have no potential conflicts of interest.
Funding Statement
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF- 2022R1I1A1A01071569).
Acknowledgements
Figures created with Biorender.com.

