The spectrum of microvascular patterns in adult diffuse glioma and their correlation with tumor grade
Article information
Abstract
Background
Primary brain tumors constitute the leading cause of cancer-related mortality. Among them, adult diffuse gliomas are the most common type, affecting the cerebral hemispheres and displaying a diffuse infiltrative pattern of growth in the surrounding neuropil that accounts for about 80% of all primary intracranial tumors. The hallmark feature of gliomas is blood vessel proliferation, which plays an important role in tumor growth, tumor biological behavior, and disease outcome. High-grade gliomas exhibit increased vascularity, the worst prognosis, and lower survival rates. Several angiogenic receptors and factors are upregulated in glioblastomas and stimulate angiogenesis signaling pathways by means of activating oncogenes and/or down-regulating tumor-suppressor genes. Existing literature has emphasized that different microvascular patterns (MVPs) are displayed in different subtypes of adult diffuse gliomas.
Methods
We examined the distribution and biological characteristics of different MVPs in 50 patients with adult diffuse gliomas. Haematoxylin and eosin staining results, along with periodic acid–Schiff and CD34 dual-stained sections, were examined to assess the vascular patterns and correlate with different grades of diffuse glioma.
Results
The present observational study on adult diffuse glioma evaluated tumor grade and MVPs. Microvascular sprouting was the most common pattern, while a bizarre pattern (type 2) was associated with the presence of a high-grade glioma. Vascular mimicry was observed in 6% of cases, all of which were grade 4 gliomas.
Conclusions
This study supplements the role of neo-angiogenesis and aberrant vasculature patterns in the grading and progression of adult diffuse gliomas, which can be future targets for planning treatment strategies.
Primary brain tumors constitute one of the leading causes of cancer-related mortality [1]. Brain tumors have a great impact on the socio-economic life of patients and their families, treatment effects, and healthcare costs. Diffuse gliomas are the most common type of malignant brain tumor according to the Central Brain Tumour Registry of the United States [2], boasting an astrocytic origin in more than 75% of cases, with glioblastomas being the most common among all diffuse gliomas. Adult diffuse gliomas generally affect the cerebral hemispheres and show a diffuse infiltrative growth pattern into the surrounding neuropil [3]. According to the World Health Organization (WHO)’s classification of tumors of the central nervous system, adult diffuse gliomas can be subdivided into three types based on their histomorphological features and genetic makeup, as follows: astrocytoma, isocitrate dehydrogenase (IDH)-mutant; oligodendroglioma, IDH mutant and 1p/19q–co-deleted; and glioblastoma, IDH–wild-type [4]. They may also be further segregated into distinct histological grades, ranging from WHO grades 1–4, on the basis of a well-defined morphology, including cellularity, nuclear atypia, mitotic activity, microvascular proliferation, and necrosis [5]. Affected patients have poor clinical outcomes, especially those with primary glioblastomas, in whom the average survival time is about 12–15 months after diagnosis [6]. The latest WHO classification of brain tumors has incorporated molecular parameters into the diagnosis of diffuse gliomas [7]. Angiogenesis plays a pivotal role in malignant transformation, and its dysregulation leads to the formation of a leaky vascular network of tumor-associated capillaries, promoting survival and proliferation of the tumor cells [8]. Gliomas are characterized by an increase in blood vessel formation, which plays an important role in tumor growth, tumor biological behavior, and disease outcome. High-grade gliomas exhibit increased vascularity, the worst prognosis and reduced survival rates. A hypothesis formulated by Weidner et al. [9], which suggested tumor growth to be an angiogenesis-dependent phenomenon, has received strong scientific support. The process of neovascularisation is multidimensional, including inflammation, the release of angiogenic cytokines, vasodilatation, and increased tumor metabolism [10]. Recently, it was postulated that the tumor vasculature is not always derived from endothelial cell proliferation and the sprouting of new capillaries but also from alternative vascularisation mechanisms like vascular co-option and vasculogenic mimicry (VM) [11]. In the present study, the authors analyzed various microvascular patterns (MVPs) in adult diffuse gliomas [12]. The degree of angiogenesis affects tumor biology and invasiveness as well as aggressive behavior. Microvascularisation is of great significance in glioma development and malignant transformation. Microvascularity is highly correlated with the grading and subtyping of diffuse gliomas and contributes as one of the most important histological features. Abnormal angiogenesis reportedly leads to proliferation, survival, and metastasis of tumor cells [13,14]. The study also examined the association of various angiogenic patterns with tumor grade.
MATERIALS AND METHODS
This cross-sectional study was carried out on 50 patients who were histopathologically diagnosed with adult diffuse gliomas between January 2019 and June 2022 in the Department of Pathology and Lab Medicine. Informed consent was obtained from all patients according to the research proposal approved by the institutional ethics committee. All cases of adult diffuse glioma included were WHO central nervous system (CNS) grade 2–4 gliomas [7]. At least one representative formalin-fixed, paraffin-embedded block was identified from each case for performing manual immunohistochemistry (IHC) by CD34 QB end 10, mouse monoclonal antibody. Sections were collected on positively charged hydrophobic slides, and IHC was performed manually in each case. IHC is a process in which various antigens can be detected within a tissue sample using an unlabelled primary antibody capable of binding those antigens of interest with high specificity. A multiepitope retrieval system (cat No. MERS-3.1) and the PolyExcel HRP/DAB detection system kit (both Pathn-Situ Biotechnologies, Hyderabad, India) were used for antigen retrieval and IHC staining, respectively.
CD34–periodic acid–Schiff dual staining
After conventional CD34 IHC staining, the last step of counterstaining with hematoxylin was skipped and the tissue was instead stained with periodic acid–Schiff (PAS) stain for 5–10 minutes, which was followed by incubation in Schiff’s reagent for another 30 minutes and then counterstaining with Mayer’s hematoxylin for 1 minute. The slides were then mounted with DPX mounting media and viewed under a light microscope (Olympus, Tokyo, Japan) to detect CD34 and PAS stain as dual magenta pink and brown signals. Adult liver tissue was used as a positive control and stained appropriately. During staining, CD34, being a pan-endothelial marker, will highlight the true vessels taking part in angiogenesis, while PAS stain will reveal the basement membrane of vessels and vascular mimicry [15,16].
MVP analysis
MVPs were analyzed in cases of adult diffuse glioma as per the criteria defined by Chen et al. [17]. Five different categories of MVPs were defined. Anti-endothelial antibody CD34 alone can categorize the four types of microvascular proliferations like microvascular sprouting (MS), microvascular clustering (MC), vascular garland (VG), and glomeruloid tuft (GT) while categorizing VM the duel staining of CD34-PAS is essential. The MS display simple vessels with delicate capillary like vessels with a lumen. Vascular clusters (VCs) are distinct focal aggregations of vessels without connective stroma. VGs are clustered vessels arranged in a garland-like formation with/without connective stroma. GTs are clustered vessels embedded within a connective stroma with a glomerulus-like appearance (containing 15–100 nuclei). Finally, VM is a phenomenon defined by the presence of vascular channels lined by cells that present negative for endothelial markers but with PAS positivity. To reduce the interobserver variability and assure reproducibility, multi-hotspot assessment was carried out by two observers independently.
Statistical analysis
The presentation of categorical variables used numbers and percentages, while qualitative variables are given using mean ± standard deviation values. For the comparison of qualitative variables that were normally distributed, the Fisher’s exact test was used to assess the association of two independent variables being a small sample size of 50. The p-value was then calculated, with p<.05 considered to be statistically significant. The analysis of data was completed with the use of the Statistical Package for Social Sciences (SPSS) software ver. 25.0 (IBM Corp., Armonk, NY, USA).
RESULTS
An ambispective, descriptive observational study was carried out on a total of 50 histologically proven cases of adult diffuse glioma. The mean age of included patients at the time of diagnosis was 43.43 years (range, 21 to 65 years), and the male-to-female ratio was 1.5:1.0, showing a male predominance. Tumors were found most commonly to involve the frontal lobe, followed by the temporal lobe, while a midline location was observed in three cases. All adult diffuse gliomas were classified based on morphology and assigned a histological grade; ultimately, it was observed that most patients (n=31) had grade 4 gliomas, followed by grade 2 (n=12) and grade 3 (n=7) gliomas, respectively.
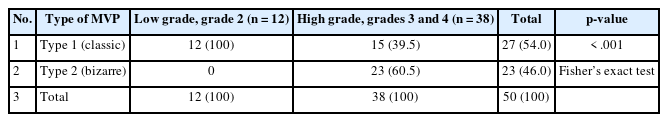
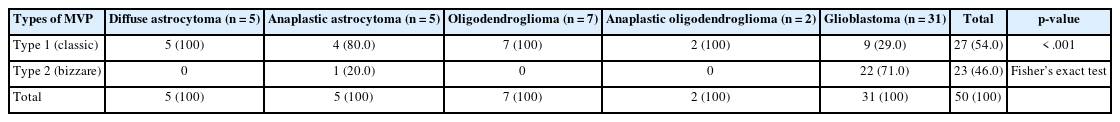
The examination of MVPs revealed MS to be the most common pattern, while VCs were the least common pattern. VGs were observed in 8% of cases, while GTs were evident in 19 out of 50 cases (38.0%) (Fig. 1). A rare pattern of VM was noticed in just three of 31 cases of grade 4 glioma (Fig. 2) on dual staining (Table 1). The MVPs were further categorized into type 1 (MS and MC) and type 2 (VG and GT) cases, respectively. The authors then further studied the association of these pattern types with tumor grade. Ultimately, it was found that the type 1 patterns were more commonly associated with lower-grade gliomas, while type 2 patterns were maximally seen in patients with high-grade gliomas (Table 2). This association was highly statistically significant (p< .001). The authors further continued to explore the association of MVPs with histological types in adult diffuse glioma (Table 3). It was noted that patients with glioblastomas displayed type 2 patterns in 22 out of 31 cases (70.97%), while diffuse astrocytoma and oligodendroglioma (grade 2) revealed type 1 patterns in 5/5 and 7/7 cases, respectively. The association of MVPs with various histological types was also highly significant (p<.001).

(A) Microvascular sprouting: simple vessels which are delicate capillary-like with a defined lumen. (B) Vascular garlands: clustered vessels arranged in garland like formation, with/without connective stroma. (C) Vascular clusters seen as focal aggregations of vessels without the connective stroma. (D) The glomeruloid tufts: clustered vessels embedded within the connective stroma with a glomerulus-like appearance (CD34 immunohistochemical staining).

Vasculogenic mimicry seen as vascular channels lined by cells, negative for endothelial markers, but exhibit periodic acid–Schiff positivity (CD34–periodic acid–Schiff dual staining).
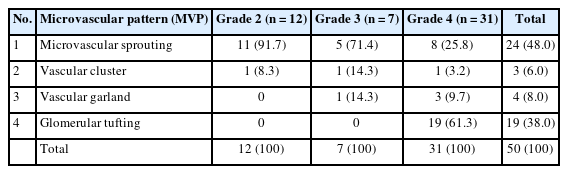
Distribution of various MVPs and their correlation with tumor grade amongst patients with adult diffuse gliomas (n = 50)
DISCUSSION
Primary brain tumors are among the most prevalent causes of cancer-related death [18]. Gliomas account for 40%–50% of all intracranial tumors, and about half of all gliomas in adults are glioblastomas [19]. Pathologically, gliomas include a range of CNS tumors originating from glial cells. Adult diffuse gliomas are defined as infiltrating glial tumors of the CNS that infiltrate the surrounding neuropil and stand as the most aggressive CNS tumors. Angiogenesis is important in the pathogenesis of a variety of non-pathological as well as pathological conditions, including malignancies [20,21]. Weidner et al. [9] first proposed that tumor growth and metastasis are dependent on angiogenesis and suggested that blocking angiogenesis could be the key to blocking the progression of the disease. Glioblastomas are highly vascularised tumors, and microvascular proliferation holds an integral role in the disease progression of low–grade gliomas to glioblastomas. The vascular networks in adult diffuse glioma shows heterogeneity in morphology as well as in the mechanisms leading to their formation and development [22]. The evidence of angiogenesis plays a critical role in biological behavior and ultimately the patients’ prognosis. This has instigated more research on the basic mechanisms of vascularization. For more than three decades, sprouting angiogenesis was considered the exclusive mechanism of vascularisation in tumors. However, several additional mechanisms, such as intussusceptive angiogenesis, vessel co-option, glomeruloid angiogenesis, recruitment of bone marrow–derived endothelial progenitor cells and VM, have been identified [23,24].
Growing tumors have been found to extend their blood supply and exhibit increased oxygen and nutritional demands by the following mechanisms: formation of new capillary buds from pre-existing vessels (sprouting), lining along the pre-existing blood vessels (vessel co-option), migration of angioblasts/endothelial progenitor cells from bone marrow under the influence of growth factors (also known as vasculogenesis), splitting of preexisting vessel with the formation of connective columns (tissue pillars) in the vascular lumen and intussusceptive microvascular growth remodeling of existing vasculature to form complex glomeruloid bodies (glomeruloid angiogenesis) [24,25].
The present study of 50 patients with adult diffuse gliomas included individuals with a wide age range of 21–65 years. Men were affected more so than their female counterparts in a ratio of 1.5:1.0. The most common pattern exhibited in our study was the MS pattern, followed by the GT, VG, and VC patterns, while VM was least common. These findings are in coherence with those of Jha et al. [16], who studied MVPs using CD34, CD31, and F VIII in 24 cases of glioblastoma and observed MS to be the most common pattern, being present in all 24 cases. The other patterns, in decreasing order, were VC, VG, GT, and VM, respectively. These bizarre microvascular patters are observed in variable combinations in adult diffuse glioma.
These authors additionally noted the presence of ‘classic’ (capillary-like) and ‘bizarre’ (GT, VCs, and vascular garlanding) vascular patterns, along with VM, in their cases of glioblastoma, and suggested that MS and simple vessels form the predominant pattern in these low-grade glial tumors.
In the present study, MS was more commonly seen in cases of grade 2 gliomas, while VCs were noticed across cases of all grades. Meanwhile, VGs were observed more commonly in grade 4 cases, and GTs were present in all grade 4 glioma cases. Our study also noted bizarre vascular patterns composing up to 46% of the total MVPs found in most of the cases of adult diffuse glioma.
Chen et al. [17] examined 78 cases of glioblastoma in their study for all five types of MVP, reporting that type 1 MVPs totaled 71.8% and type 2 MVPs totaled 28.2%. These authors concluded that there exist significant differences in prognosis according to MVP type; notably, the cases with type 2 patterns exhibited poorer progression-free and overall survival rates than those with type 1 patterns.
According to the study by Yue and Chen [15], the poor prognosis and MVPs of glioblastoma cases are of serious concern to many clinicians and researchers. However, very few studies to date have examined the correlation between microvascular niche patterns (MVNPs) and proteomic distribution. In their study, CD34 immunofluorescence staining and matrix-assisted laser desorption ionization mass spectrometry imaging technology were used to investigate protein distributions in MVNPs. Based on such characteristics, MVNPs were divided into two types by cluster analysis—namely, type I (classic patterns), composed primarily of MS and VC cases, and type II (bizarre patterns), composed primarily of VG and GT cases. Survival analysis indicated that MVNP type, such as classic or bizarre pattern, to be an independent prognostic factor for progression-free and overall survival among patients with glioblastoma (Table 4) [26].
The present study documented type 1 patterns in 54% and type 2 patterns in 46% of cases, respectively. Type 1 proliferation was associated with low-grade gliomas, while type 2 MVPs were seen in association with high-grade gliomas in our study. It was postulated that tumor cells themselves may de-differentiating and acquire an endothelial phenotype, forming a PAS positive vascular pattern called VM. The VM pattern was first described in cases of uveal melanoma. Intussusceptive microvascular growth (IMG) has been found to be a quicker way of increasing the complexity of the tumor microvessel network, independent of endothelial cell proliferation. It has been found that this process becomes relevant once the sprouting of blood vessels has begun. It has been suggested that the absence of endothelial cell proliferation in IMG implies that neovascularisation by this mechanism would be resistant to angiosuppressive treatment. Microvascular proliferation has since been observed in a variety of solid tumor, such as carcinoma of the breast, prostate, ovaries, lungs, synovial sarcoma, rhabdomyosarcoma, pheochromocytoma, and glioblastomas. The discovery of VM has challenged the hypothesis that angiogenesis is the only means by which tumors acquire a blood supply [27]. In a recent study, Jha et al. [16] proposed the presence of vascular mimicry to be an adverse prognostic factor for postoperative survival in newly diagnosed cases of glioblastoma.
VM was found in 6% of patients with adult diffuse gliomas in the current study, all of whom had grade 4 tumors. In contrast, Jha et al. [16] reported 50% of cases in their study had VM, and 38.1% of cases in the study by Liu et al. [28] and 28.75% of cases in the study by Wang et al. [29] also had the VM pattern, respectively.
Chen et al. [17] in their study found that the proportion of cases with the VM pattern was significantly low relative to the proportions of other MVP types. They also observed a potential relationship between the expressions of Ki-67 and p53 and microvascular heterogeneity. These findings led us to speculate whether vascular parameters have significance apart from influencing the tumor grade. Several trials have evaluated the impact of anti-angiogenic treatment on the overall survival of glioblastoma patients but offered inconsistent results. Alternate mechanisms of vascularisation operating during tumor proliferation and growth may be responsible for the failure of anti-angiogenic therapy. It was further hypothesized that multiple vascularisation mechanisms and angiogenic signaling pathways exist, and the inhibition of any single signaling pathway may trigger the onset of alternative vascularisation mechanisms.
Although there is significant heterogeneity among MVPs, in cases of adult diffuse glioma, specific histomorphological patterns of MVPs allowed us to classify these tumors into two categories. Notably, we found that type 2 patterns, including VM, were associated with higher grades of glioma. This signifies and further supplements the role not only of neo-angiogenesis but also the aberrant or abnormal vasculature in disease progression and could help realize future targets for planning treatment strategies.
One of the important limitations of the present study is that, while categorizing adult diffuse gliomas, molecular characterization or mutational analysis was not taken into account due to resource limitations. This study enrolled cases of adult diffuse gliomas and did not include pediatric gliomas. Therefore, the results of the current study are totally based on the histomorphological grading of adult diffuse gliomas and the correlation with microvascular proliferations. We recommend that further research should gather more thoughts or any additional information that may be available when molecular classification is taken into account for the integrative diagnosis of adult diffuse glioma.
Notes
Ethics Statement
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of University B (Date.24/02/21/No.2020/PG/July/32). Written informed consents were obtained.
Availability of Data and Material
The datasets generated or analyzed during the study are available from the corresponding author on reasonable request.
Code Availability
Not applicable.
Author Contributions
Conceptualization: VW, S. Data curation: S, AS. Formal analysis: S, TS, AA. Funding acquisition: S, VW. Investigation: TS, DJ, VW. Methodology: S, AS. Writing—original draft: S, AS, VW. Writing—review & editing: DJ, AA, VW. Approval of final manuscript: all authors.
Conflicts of Interest
The authors declare that they have no potential conflicts of interest.
Funding Statement
No funding to declare.