What’s new in adrenal gland pathology: WHO 5th edition for adrenal cortex
Article information
Abstract
The 5th edition of WHO Classification of Endocrine and Neuroendocrine Tumors (2022) introduced many significant changes relevant to endocrine daily practice. In this newsletter, we summarize the notable changes to the adrenal cortex based on the 5th edition of the WHO classification [1].
NEW CHAPTERS
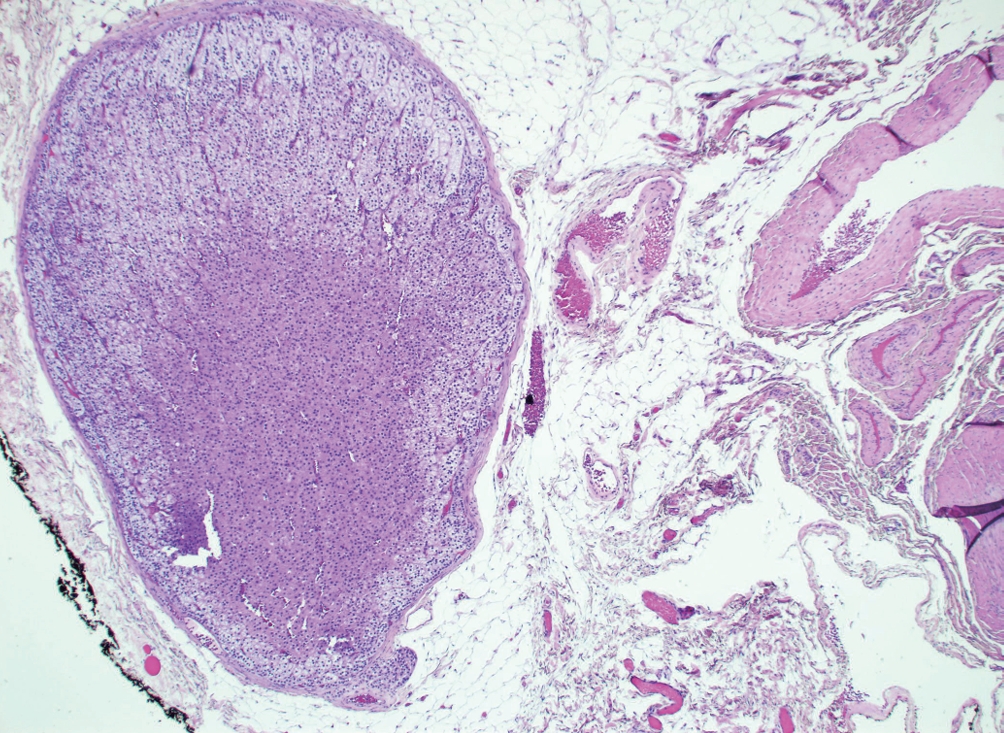
Adrenal ectopia
• Benign adrenal tissue in an aberrant location (Fig. 1).
• Includes “adrenal rests,” which are aligned with normal embryogenesis (e.g. kidneys or gonads), and “adrenal cortical choristoma,” which are in locations not aligned with embryogenesis.
• The vast majority are incidental findings, contain only adrenocortical cells, and express SF1.
• Rarely, adrenal cortical neoplasms may arise from ectopic adrenal tissue.
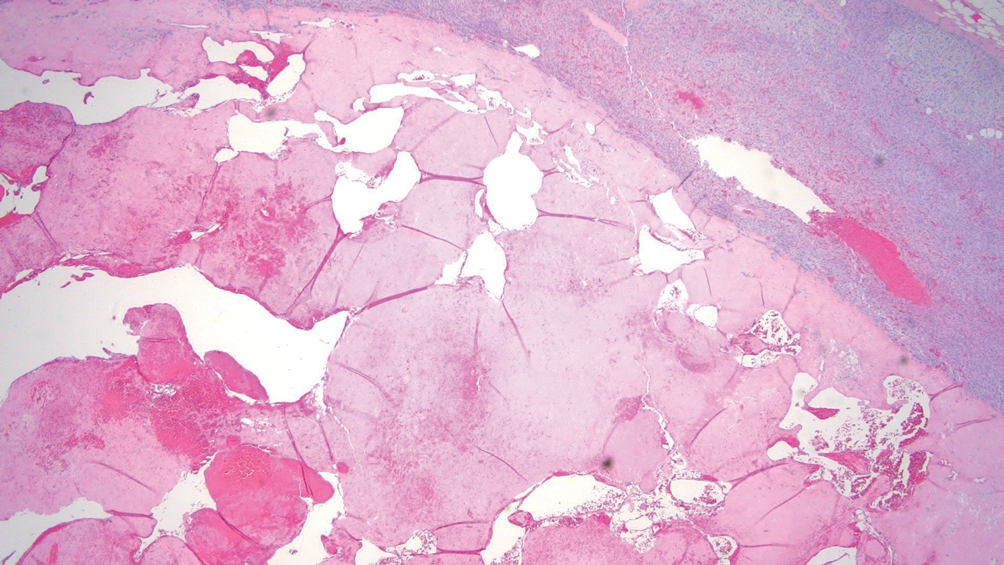
Adrenal cysts
• Benign, circumscribed, fluid-containing masses, which are divided into 4 subtypes:
° Pseudocysts: most common (>60% of adrenal cysts), do not have a cell lining, occur following trauma, hemorrhage, or infection including COVID-19 (Fig. 2).
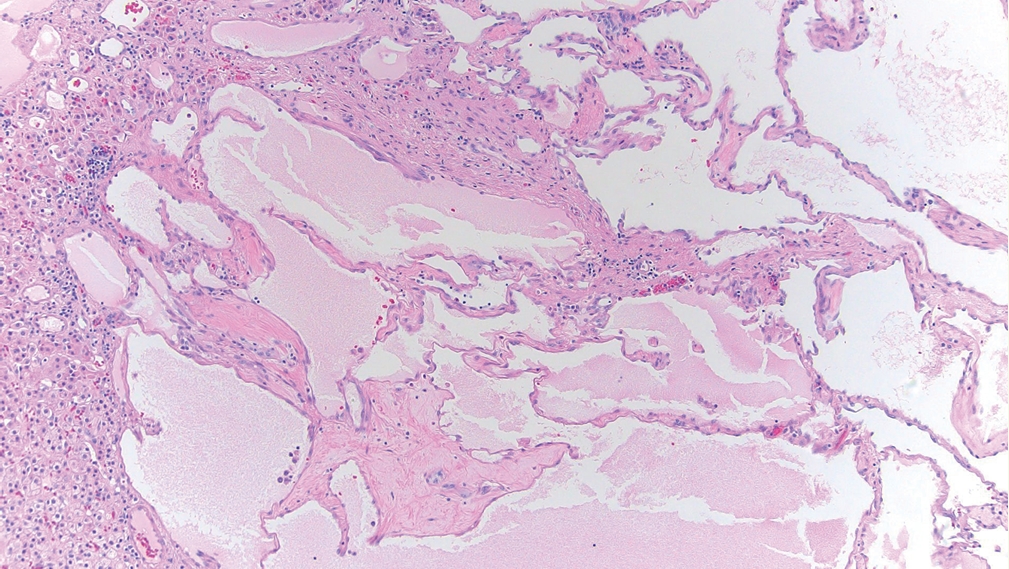
° Endothelial (vascular) cysts: lined by endothelium, occur as a malformation or as part of recanalization (Fig. 3).
° Epithelial cysts: lined by mesothelium, occur as an inclusion cyst of embryologic remnants.
° Parasitic cysts: rare, uni/multi-loculated cysts with a fibrous wall and clear contents.
• A broad differential ought to be considered since cystic changes can occur in adrenocortical adenoma/carcinoma, pheochromocytoma, hemangioma, renal cell carcinoma, metastases, etc.
UPDATED CLASSIFICATION OF ADRENAL CORTICAL PROLIFERATIONS
Adrenal cortical hyperplasia
• Cortical zonation is intact as this is a physiologic response, rather than a clonal proliferation.
• Three types of true hyperplasia:
° Congenital adrenal hyperplasia (CAH): caused by mutations in genes encoding enzymes of steroid production.
° ACTH/CRH dependent diffuse hyperplasia: caused by Cushing’s disease, ectopic secretion, chronic stress.
° Diffuse zona glomerulosa hyperplasia: idiopathic.
Adrenal cortical nodular disease
• Group of sporadic or germline nodular clonal proliferations:
° Sporadic nodular adrenocortical disease:
- Formerly known as “nodular adrenal cortical hyperplasia”.
- <10 mm non-functional adrenocortical nodules.
° Bilateral micronodular adrenocortical disease:
- Occurs more commonly in children and young adults (<30 years old).
- <10 mm bilateral adrenocortical nodules, could be pigmented.
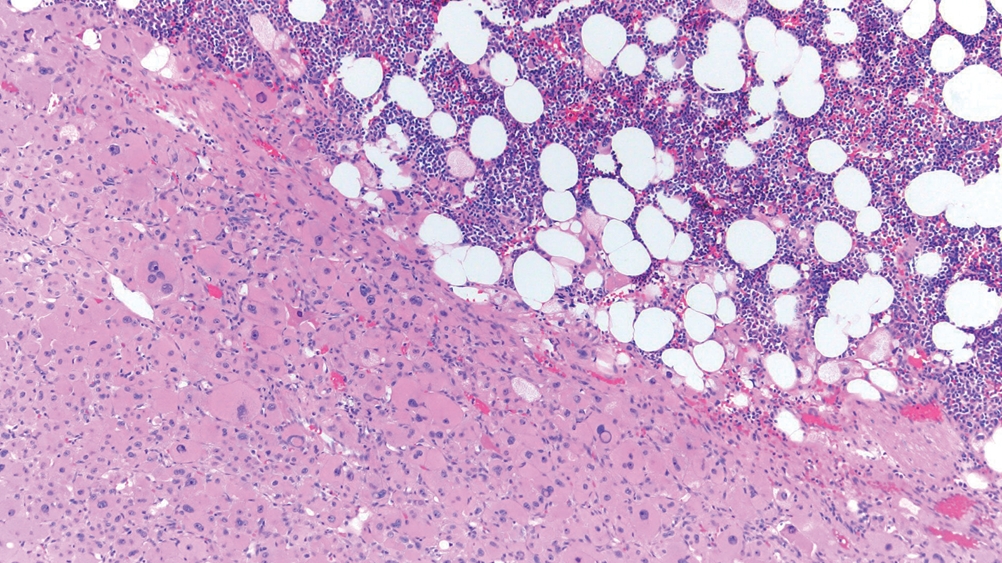
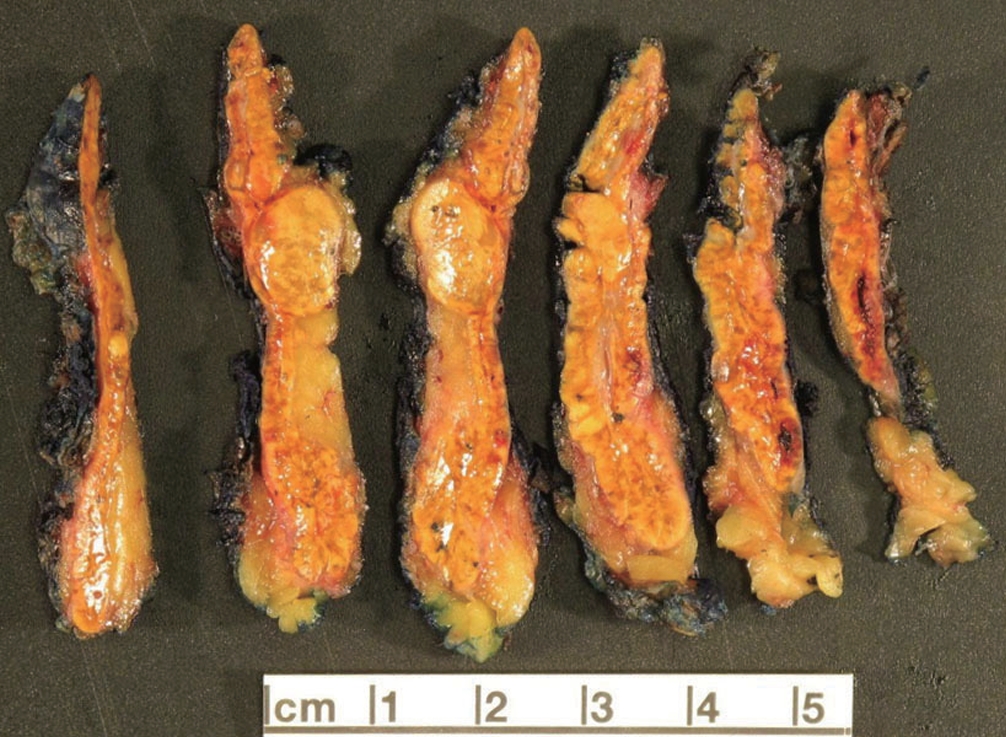
° Bilateral macronodular adrenocortical disease:
- More frequent in adults.
- >10 mm bilateral nodules (Fig. 5).
- Formerly known as “primary bilateral macronodular adrenal cortical hyperplasia” or “ACTH-independent macronodular adrenocortical hyperplasia”.
• Bilateral micronodular and macronodular forms of adrenocortical nodular disease typically contribute to hypercortisolism and are often associated with germline variants in specific susceptibility genes (PRKAR1A, PRKACA, PDE11A, PDE8B, ARMC5, MEN1, etc).
• The former designation “hyperplasia” is discouraged as it is a physiological reaction to elevated ACTH levels. The term should not be used to describe multifocal nodules resulting from clonal expansions.
Primary aldosteronism (PA)
• Primary aldosteronism (Conn syndrome) is a leading cause of secondary hypertension characterized by aldosterone overproduction and suppression of the renin-angiotensin system.
• HISTALDO Classification combines CYP11B2 (aldosterone synthase) immunohistochemistry and morphologic features to predict the risk of biochemical recurrence.
• “Classic” histology: 5% recurrence risk
° Aldosterone-producing adrenal cortical carcinoma (APACC)
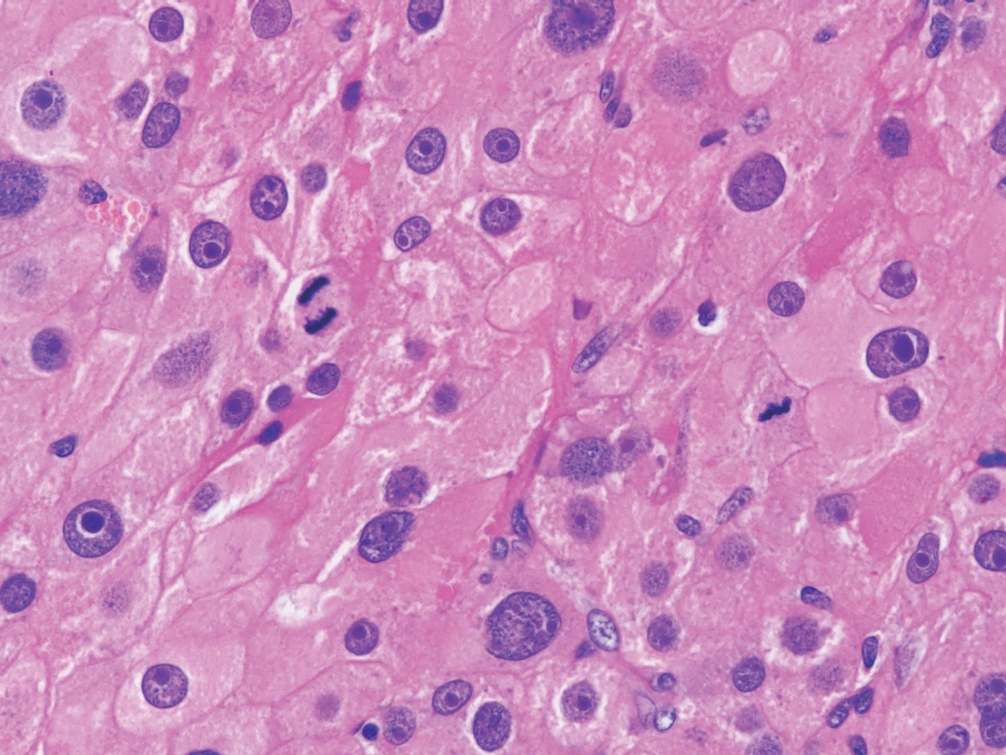
° Aldosterone-producing adrenal cortical adenoma (APA) (Fig. 6): solitary, > 10 mm, CYP11B2 diffuse reactivity.
Functional aldosterone-producing adrenal cortical adenoma with predominantly vacuolated cells and scattered pleomorphism.
° Aldosterone-producing nodule (APN): solitary, < 10 mm, CYP11B2 gradient reactivity, emphasizing increased intensity at the outer part of the nodule.
• “Non-classic” histology: 42% recurrence risk
° Aldosterone-producing micronodule (APM): < 10 mm, may not be recognized on H&E but is highlighted with CYP11B2 (gradient reactivity).
° Multifocal aldosterone-producing nodules (APNs).
° Aldosterone-producing bilateral diffuse hyperplasia (APDH).
Adrenal cortical adenoma
• Adrenocortical neoplasm that lacks morphologic features of malignancy.
• Features worrisome for malignancy: vascular invasion, tumor necrosis, atypical mitotic figures, increased mitotic activity (>5 mitoses per 10 mm2), loss of reticulin framework.
• Usually unilateral and solitary; can be nonfunctional or hormonally active.
CLASSIFICATION OF ADRENAL CORTICAL CARCINOMAS, MAIN SUBTYPES, ANCILLARY STUDIES & GRADING
Adrenal cortical carcinoma
• Malignant adrenocortical neoplasm; can be nonfunctional or hormonally active.
• Adrenal masses associated with virilization or feminization are clinically highly worrisome for malignancy.
• In addition to conventional adrenal cortical carcinoma, three morphologic subtypes exist:
° Oncocytic: oncocytic cells in >90% of tumor; extensive sampling is required to better quantify the oncocytic component.
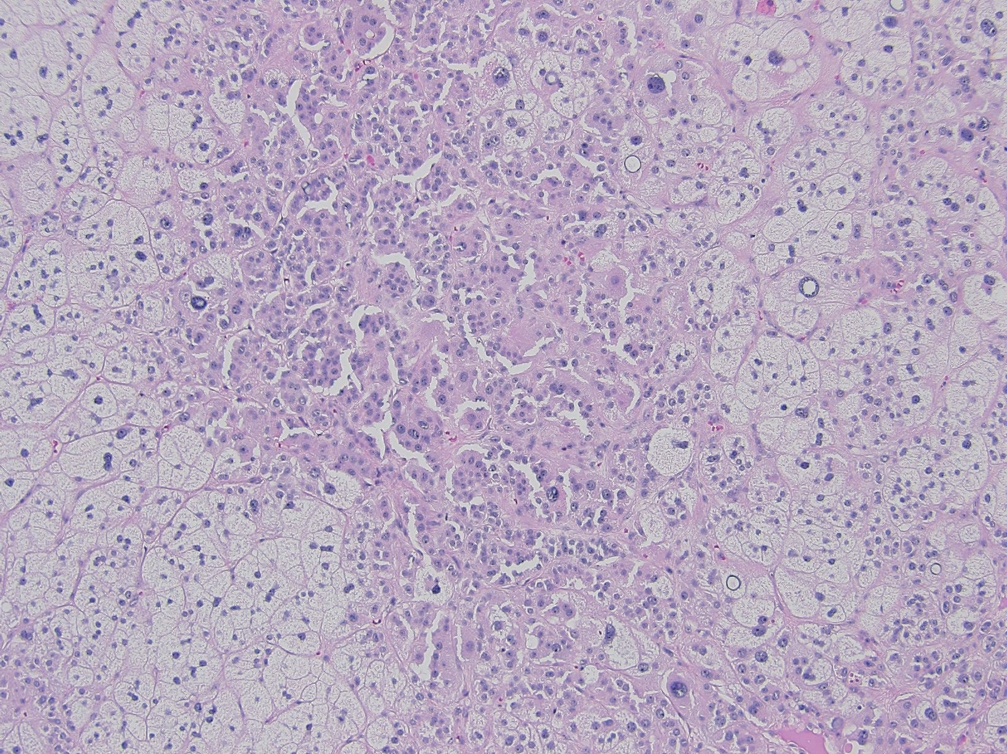
° Myxoid: prominent extracellular mucin deposition; poor prognosis (Fig. 7).
° Sarcomatoid: resembles sarcomatoid carcinomas of other organs; poor prognosis.
• Vascular invasion is an important diagnostic and prognostic tool in assessment of malignancy. It is assessed at the intersection of tumor and adrenal capsule or beyond the capsule where tumor cells are seen invading through the vessel wall and forming a thrombus/fibrin-tumor complex.
• Many scoring criteria are available for diagnosing adrenal cortical carcinoma and risk stratification; their use depends on morphologic subtype and patient age:
° Weiss score, modified Weiss, and Helsinki multiparameter scores.
° Lin-Weiss-Bisceglia system was developed to evaluate oncocytic adrenal cortical neoplasms.
° Wieneke system is used for assessing pediatric adrenal cortical neoplasms.
° Reticulin algorithm requires an altered reticulin network, in association with any one of the following: increased mitotic rate (>5 mitoses per 50 high-power fields [HPF]), tumor necrosis, or vascular invasion (Fig. 8).
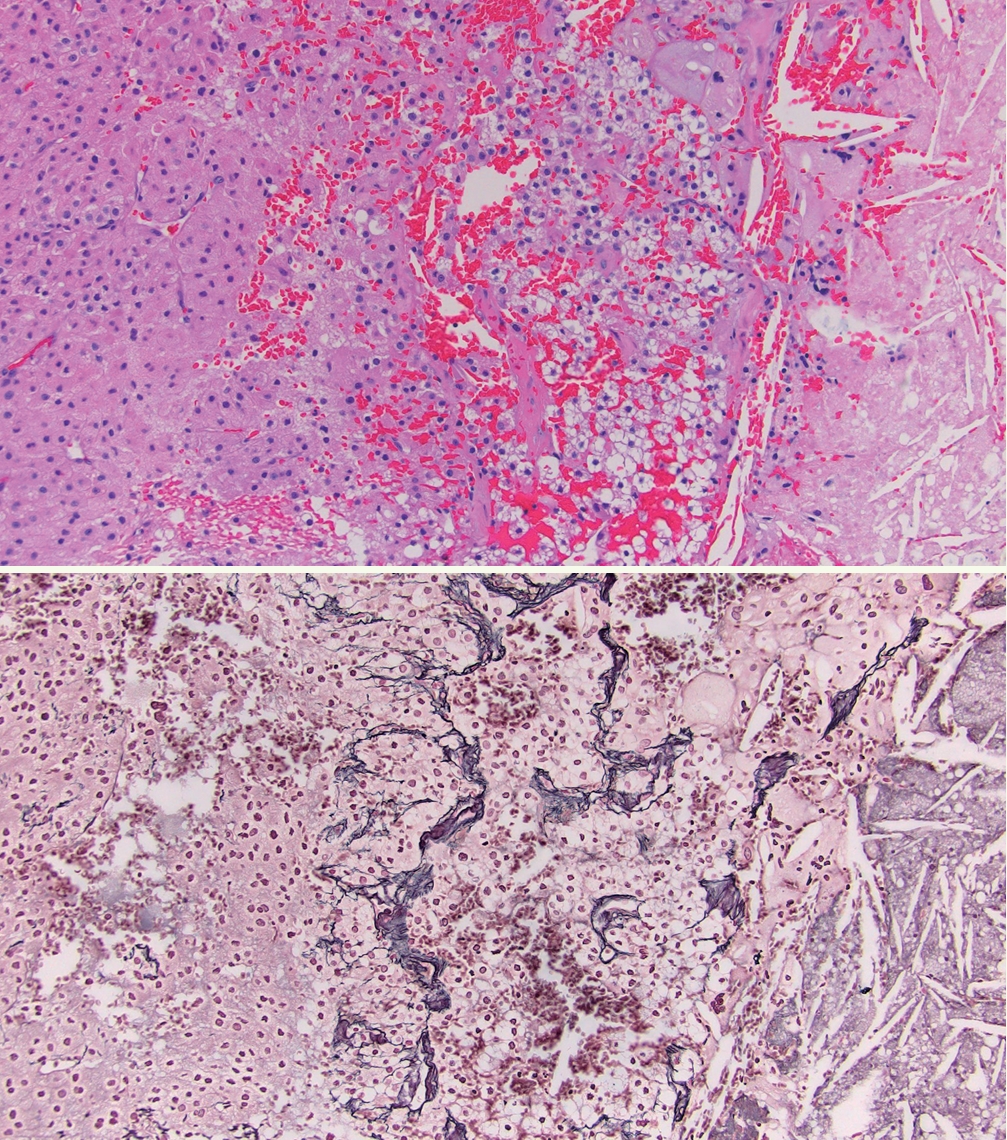
A: Adrenal cortical carcinoma with tumor necrosis (right of image). B: Adrenal cortical carcinoma with a disrupted reticulin framework.
• A simplified approach via the reticulin algorithm has gained popularity given its high reproducibility and applicability to all morphologic subtypes.
• Following use of the reticulin algorithm, a carcinoma is subsequently classified as low-grade or high-grade based on mitotic activity (high grade if >20 mitoses/50 HPF) (Fig. 9).
• Diagnostic and prognostic biomarkers:
° p53: overexpression or global loss may be identified in high-grade areas.
° Beta-catenin: nuclear overexpression is a poor prognostic sign.
° IGF2: used as a diagnostic tool given that paranuclear granular expression is found in ≈80% of adrenal cortical carcinomas.
° Ki67: recommended to specify the Ki67 labeling index for all adrenal cortical carcinomas via manual count or automated image analysis, and to document the methodology used. Carcinomas typically label >5%, and the index matters in terms of prognosis.
Meet the Authors
Dr. Carol N. Rizkalla is a second-year AP/CP pathology resident at the University of Washington. She has been an author for PathologyOutlines.com since 2022, immersing herself in a variety of genitourinary topics. Regarding her future career, she is passionate about surgical pathology, with a specific focus on genitourinary and gynecologic pathology.
Dr. Tretiakova has been an author for PathologyOutlines.com since 2015, part of the editorial board since 2019, and Deputy Editor in Chief for GU Pathology since 2021. She is currently a Professor and Director of Genitourinary Fellowship and Immunohistochemistry Laboratories at the University of Washington where she primarily practices GU Pathology.