Liquid-based cytology features of pancreatic acinar cell carcinoma: comparison with other non-ductal neoplasms of the pancreas
Article information
Abstract
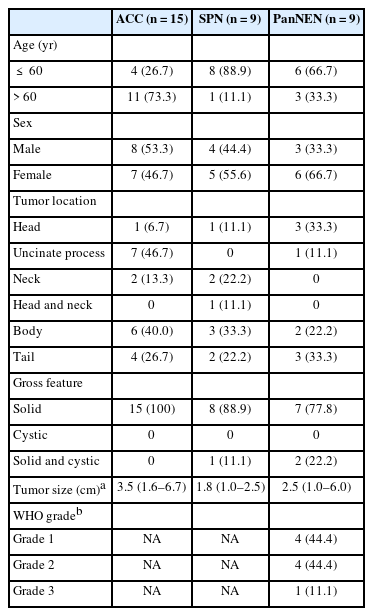
Background
Acinar cell carcinoma (ACC) is a rare malignant epithelial neoplasm, which shares many cytomorphological features with other non-ductal pancreatic neoplasms such as pancreatic neuroendocrine neoplasm (PanNEN) and solid-pseudopapillary neoplasm (SPN). Due to the relative rarity of these tumors, pathologists are less familiar with the cytological features, especially on liquid-based cytology (LBC) which has been relatively recently introduced for endoscopic ultrasound-guided fine needle aspiration specimens.
Methods
We evaluated the detailed cytological features of 15 histologically confirmed ACC (7 conventional smears [CS], 8 LBC), and compared them with the LBC features of SPN (n = 9) and PanNEN (n = 9).
Results
Compared with CS, LBCs of ACC demonstrated significantly less bloody background. All ACCs demonstrated prominent nucleoli and macronucleoli on LBC. On comparison with the LBC features of SPN and PanNEN, most ACCs demonstrated a necrotic background with apoptotic debris while PanNEN and SPN did not show these features. Acinar structures were predominantly observed in ACC, while frequent pseudopapillary structures were seen only in SPN. Prominent nucleoli and macronucleoli were only seen in ACC.
Conclusions
ACC had characteristic cytological features that could be observed on LBC preparations, such as high cellularity, necrotic/apoptotic background, nuclear tangles, acinar arrangement of cells, and macronucleoli. These findings also help distinguish ACC from PanNEN and SPN on LBC. It is important to be familiar with these features, as an accurate diagnosis on endoscopic ultrasound–guided fine needle aspiration cytology would have impact on the management of the patient.
Acinar cell carcinoma (ACC) of the pancreas is a rare malignant epithelial neoplasm accounting for up to 1% of carcinomas of the exocrine pancreas [1]. Diagnostic accuracy is critical as symptoms are nonspecific and the prognosis is poor, with a median survival of 47 months and about one-half of patients presenting with metastasis at the time of diagnosis [1-3].
Endoscopic ultrasound–guided fine needle aspiration (EUS-FNA) is a well-established diagnostic method for solid pancreatic tumors, and thus an accurate diagnosis on aspiration cytology material is essential to guide the next steps for patient management [4,5]. While conventional smear (CS) cytology was the standard method for processing EUS-FNA cytology specimens, CS preparations often result in bloody smears, dry artifacts, and crushing artifacts, which could obscure the cytologic features and result in a suboptimal diagnosis [6]. As such, there is currently increasing interest in implementing liquid-based cytology (LBC) preparations for EUS-FNA material to circumvent these innate limitation of CS specimens [6].
The cytological diagnosis of ACC is often challenging, most importantly due to the rarity of the tumor; ACC has a high rate of misdiagnosis by cytology and is often misinterpreted as other solid pancreatic neoplasms, such as pancreatic neuroendocrine neoplasms (PanNEN), solid-pseudopapillary neoplasms (SPN), and pancreatoblastoma, which demonstrate overlapping cytological features [3,4,7-10]. In addition, ACC may demonstrate scattered neuroendocrine cells in up to 40% of cases, which may falsely lead to a diagnosis of PanNEN [4]. Moreover, while immunohistochemistry may be performed on cell block specimens when available, there is also some immunophenotypical overlap between ACC with PanNEN, which adds to the diagnostic difficulty [3,11,12]. Finally, inadequate smears due to low cellularity or bloody smears may also lead to underdiagnosis of such cases [13].
While the cytological characteristics of ACC on CS have been described in the previous literature, the cytomorphology on LBC has been less well characterized [1]. The aim of this study was to evaluate the cytological features of ACC on LBC, by comparing CS and LBC features, and also by comparing the LBC cytology of ACC, SPN, and PanNEN.
MATERIALS AND METHODS
Case selection
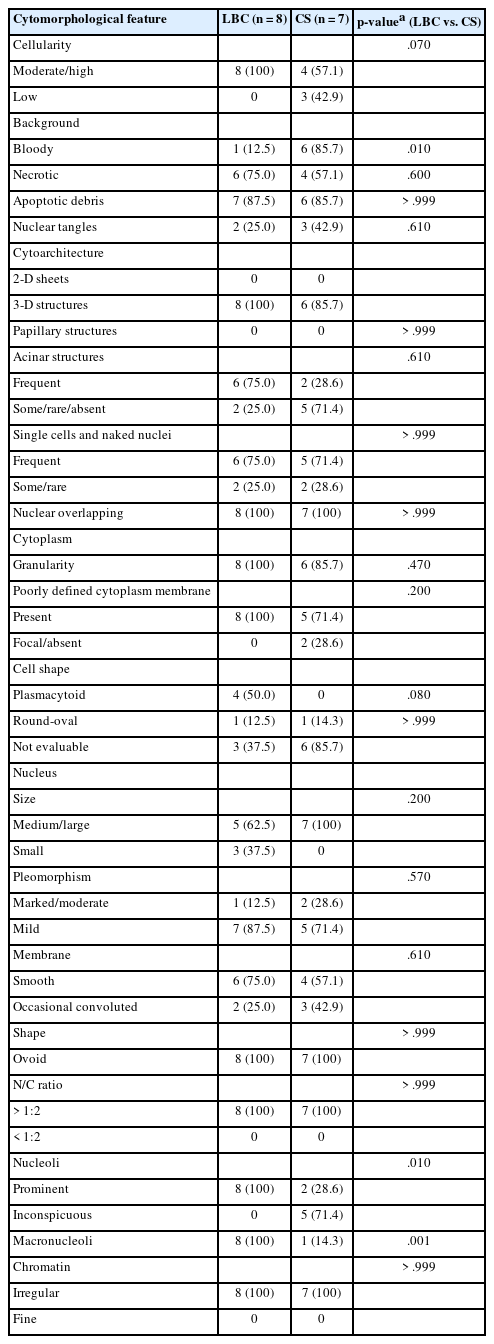
In this retrospective study, histologically confirmed cases with a final histological diagnosis of ACC over a 9-year period from 23 December 2013 to 14 July 2022 were retrieved from the pathology database of Seoul National University Hospital (SNUH) and Ulsan University Asan Medical Center. All cytology specimens were obtained by EUS-FNA using 19- or 22-gauge needles. For CS, aspirated specimens were immediately smeared onto glass slides and fixed in 95% ethanol in the endoscopy room; the slides were sent to the cytopathology laboratory for further Papanicolaou stains. For all LBC cases, the specimens were immediately suspended in preservative fluid (CytoRich Red, Thermo Scientific, Waltham, MA, USA), sent to the cytopathology laboratory and further processed on the BD PrepStain Slide Processor (Becton Dickinson, Franklin Lakes, NJ, USA) with the NON-GYN protocol.
Among these cases, a total of 15 cases (7 CS and 8 LBC) had matching preoperative EUS-FNA cytology specimens. Of these 15 cases with a final histological diagnosis of ACC, six cases were initially interpreted as “ACC”, seven cases as “carcinoma”, and two cases as “adenocarcinoma” on cytology. Immunohistochemical stains were performed for all 15 cases.
In addition, EUS-FNA LBC specimens from nine PanNENs and nine SPNs (all of which were histologically confirmed) were retrieved from SNUH, for comparison with the LBC features of ACC.
The clinicopathological features and radiological features (magnetic resonance imaging and/or abdominal computed tomography) were reviewed for all ACC, SPN, and PanNEN cases.
Cytomorphological evaluation
The cytological features of LBC and CS specimens were reviewed by two pathologists (MK, HK), and the following parameters were evaluated: (1) cellularity (high, moderate, low); (2) background (bloody or necrotic background, presence of apoptotic debris and/or nuclear tangles); (3) cytoarchitecture (2-dimensional sheets, acinar or (pseudo)papillary structures, single cells, naked nuclei, nuclear overlapping); (4) cytoplasmic features (granularity, cell membranes, cell shape); and (5) nuclear features (size, degree of pleomorphism, nuclear shape, nuclear: cytoplasmic ratio, nucleoli, macronucleoli, and chromatin pattern). The cytological features were initially evaluated independently, followed by discussion and review using a multiheaded microscope to render a final assessment.
Statistical analysis
Statistical analysis was performed by Chi-square tests and Fisher’s exact test using the R software (v3.6.1, R Foundation for Statistical Computing, Vienna, Austria). Values of p<.05 were considered statistically significant and all p-values were 2-sided.
RESULTS
Baseline characteristics
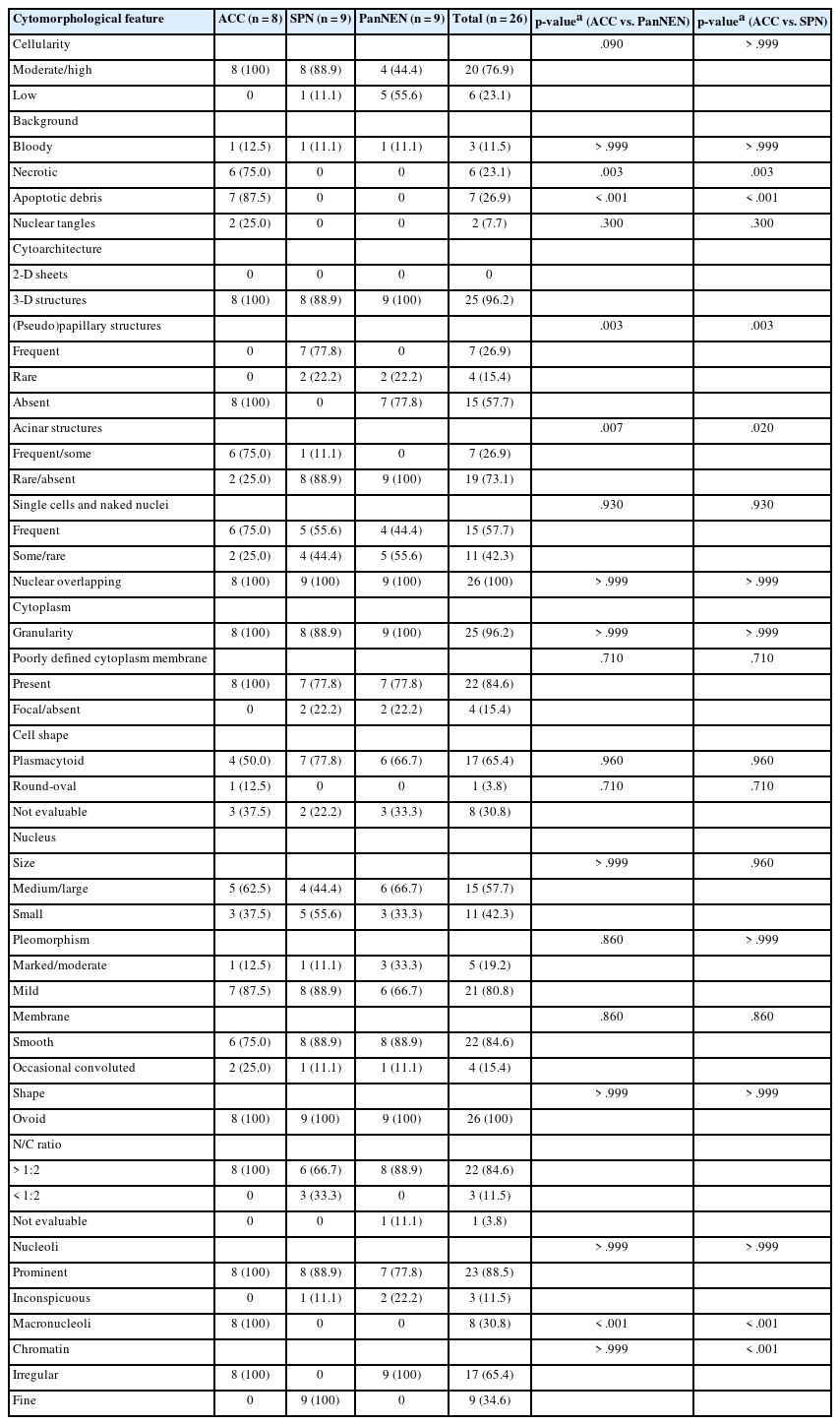
The clinicopathological details of the ACC, SPN, and PanNEN cases are summarized in Table 1. Most of the patients with SPN (8/9, 88.9%) and PanNEN (6/9, 66.7%) were younger than 60 years of age, whereas 73.3% (11/15) of patients with ACC were aged 60 or older. There was no significant predilection for location within the pancreas for all tumors, although SPNs were more commonly located in the body/tail region than in the head/uncinate process. The gross appearance was predominantly solid in all three tumors.
The initial radiological impression for the ACC cases were as follows: (1) pancreatic cancer (9/15, 60.0%), (2) “atypical pancreatic cancer” with differential diagnoses including malignant lymphoma, metastases, PanNEN and sarcoma (5/15, 33.3%), (3) and SPN (1/15, 6.7%). The most common imaging diagnosis for SPN was “SPN versus PanNEN” (6/9, 66.7%), while PanNENs were mostly classified as either “PanNEN” (4/9, 44.4%) or “PanNEN or SPN” (3/9, 33.3%) on imaging.
Comparison between LBC and CS cytology features of ACC
The detailed cytomorphological features of ACC on CS and LBC are summarized in Table 2 and Fig. 1. When cellularity was evaluated, all eight LBC cases (100%) showed moderate to high cellularity, compared to that of CS (4/7 cases, 57.1%). A bloody background was more frequently seen in CS preparations (CS, 85.7%; LBC, 12.5%; p=.010). Necrotic backgrounds were seen in both CS (57.1%) and LBC (75.0%), and apoptotic debris were frequently seen in both CS (85.7%) and LBC (87.5%) preparations. Next, we assessed the cytoarchitecture. Acinar structures was more frequently seen in LBC preparations compared with CS (CS, 28.6%; LBC, 75.0%). Notably, single cells and naked nuclei were frequently seen in ACC in both LBC (75.0%) and CS (71.4%), and nuclear overlapping was observed in all cases regardless of the type of preparation (CS, 100%; LBC, 100%).
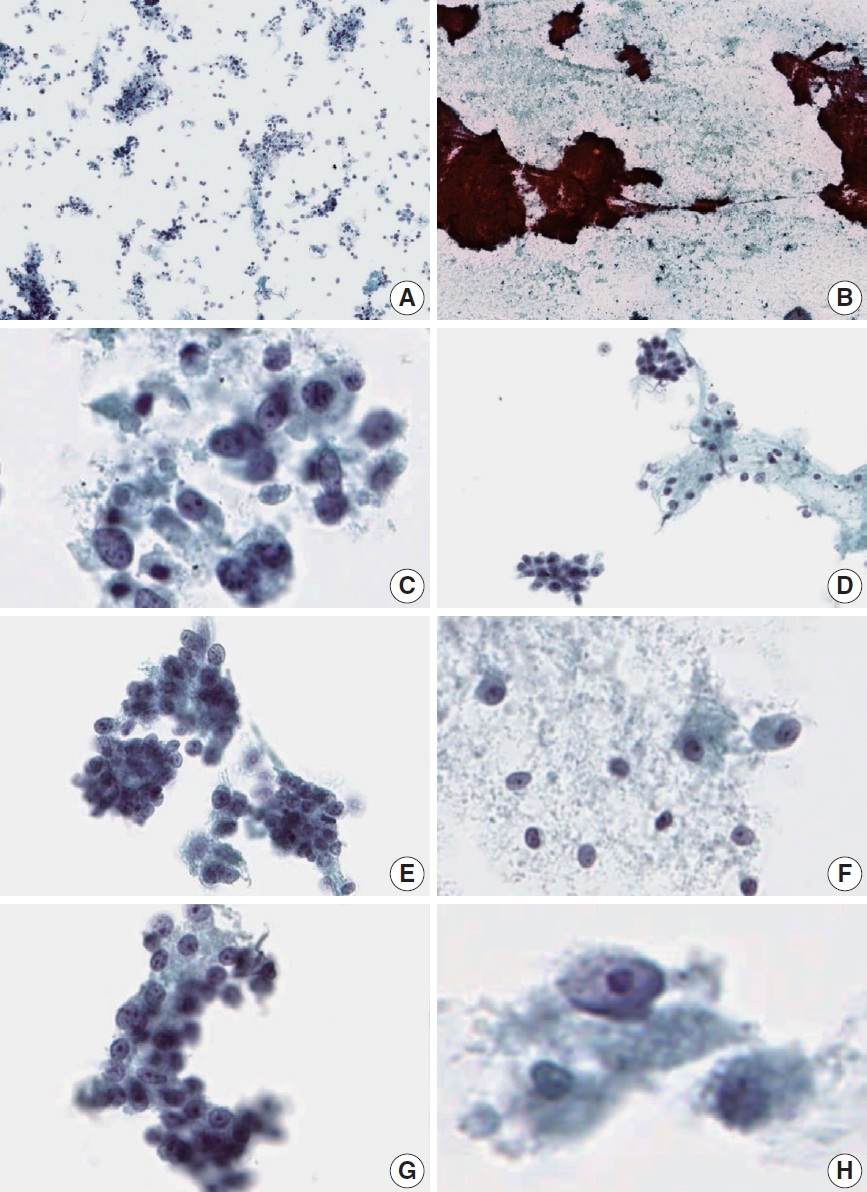
Cytologic features of acinar cell carcinoma on liquid-based cytology (LBC) and conventional smear (CS). (A) Hypercellular background (LBC). (B) Bloody background (CS). (C) Necrotic background (LBC). (D) Acinar structure (LBC). (E) Nuclear overlapping (LBC). (F) Plasmacytoid cell shape (LBC). (G) Prominent nucleoli (LBC). (H) Macronucleoli (LBC).
Cytoplasmic granularity was a common finding of ACC in both LBC (100%) and CS (85.7%). The cell borders were often blurred in ACCs. Among the cases where the cell membranes could be discerned, the ACC tumor cells often assumed a plasmacytoid cell shape on LBC. As for the nuclear features, the ACC tumor nuclei were predominantly medium-to-large in both preparations (CS, 100%; LBC, 62.5%), and high nuclear:cytoplasmic ratio and irregular chromatin were observed in all ACC cases (CS, 100%; LBC, 100%). Interestingly, prominent nucleoli and macronucleoli could be appreciated in all LBC cases of ACC, while only 28.6% (p=.010) and 14.3% (p=.001) of CS cases demonstrated these features, respectively.
In summary, compared to CS, LBC cytology of ACC was characterized by less bloody backgrounds, relatively higher cellularity, more appreciable acinar structures, and prominent (or macro) nucleoli.
Comparison of LBC features between ACC, PanNEN, and SPN
Next, we sought to compare the LBC features of ACC with those of SPN and PanNEN. The results are summarized in Table 3 and Fig. 2. ACC and SPN cases more often showed moderate to high cellularity compared to PanNENs, although not statistically significant (ACC, 100%; SPN, 88.9%; PanNEN, 44.4%). Findings that were unique to ACC were (1) a necrotic background (ACC, 75.0%; SPN, 0%; PanNEN, 0%; p=.003), (2) apoptotic debris in the background (ACC, 87.5%; SPN, 0%; PanNEN, 0%; p<.001), and (3) nuclear tangles (ACC, 25.0%; SPN, 0%; PanNEN, 0%). A bloody background was seen in a minority of ACC, SPN, and PanNEN LBCs (ACC, 12.5%; SPN, 11.1%; PanNEN, 11.1%).
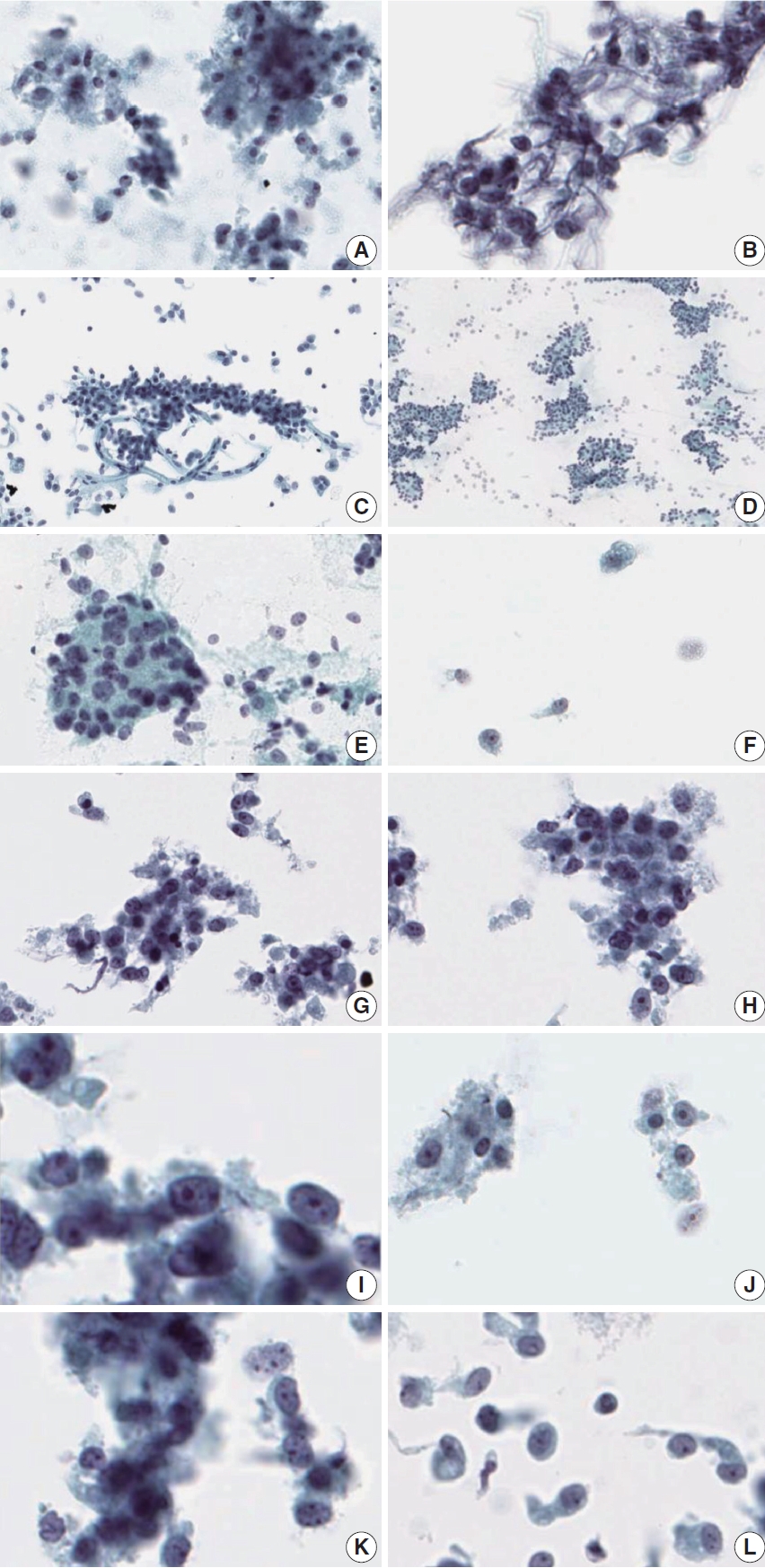
Comparison of liquid-based cytology features between acinar cell carcinoma (ACC) and solid-pseudopapillary neoplasm (SPN). Liquid- based cytology of ACC demonstrates necrotic debris in the background (A) and nuclear tangles (B). Cell clusters showing three-dimensional pseudopapillary architecture in SPN (C) and the typical acinar architecture of PanNEN (D). Some scattered cells showing singly dispersed naked nuclei (E) with occasional plasmacytoid cells (F) in ACC. Tumor cells of ACC demonstrating ovoid nuclei with mild pleomorphism (G), and irregular nuclear membranes (H). Nucleoli are prominent in ACC, with some macronucleoli (I, J). Chromatin is irregular in ACC (K), compared with the fine chromatin observed in SPN (L).
As for cytoarchitecture, the presence of frequent pseudopapillary structures was a unique feature of SPN (ACC, 0%; SPN, 77.8%; PanNEN, 0%; p=.003); in contrast, rare papillary structures (with fibrovascular cores) were observed in PanNENs (22.2%). Acinar structures, on the other hand, were more frequently observed in ACC (ACC, 75.0%; SPN, 11.1%; PanNEN, 0%; p=.007 [ACC vs. PanNEN], p=.020 [ACC vs. SPN]). Singly scattered tumor cells and naked nuclei were seen most commonly in ACC although not statistically significant (ACC, 75.0%; SPN, 55.6%; PanNEN, 44.4%).
Cytoplasmic granularity and poorly defined cell membranes were seen in all three tumor types. Plasmacytoid cell morphology was observed in 50.0%, 77.8%, and 66.7% of ACC, SPN, and PanNEN, respectively. The nuclei were ovoid in shape for all tumor types. Nuclear pleomorphism was mild in most cases (ACC, 87.5%; SPN, 88.9%; PanNEN, 66.7%). Irregular nuclear membranes were more frequently observed in ACC (25.0%) compared to SPN (11.1%) and PanNEN (11.1%), although not statistically significant. High nuclear:cytoplasmic ratio and prominent nucleoli were observed in all ACC cases, and also in the majority of SPNs (66.7% and 88.9%, respectively) and PanNENs (88.9% and 77.8%, respectively). Macronucleoli were only observed in ACCs (ACC, 100%; SPN, 0%; PanNEN, 0%; p<.001). Irregular chromatin pattern was observed in all cases of ACC and PanNEN (ACC, 100%; SPN, 0%; PanNEN, 100%) while only SPN showed fine chromatin in all cases (ACC, 0%; SPN, 100%; PanNEN, 0%, p<.001).
DISCUSSION
Although EUS-FNA cytology is currently established as the procedure of choice for diagnosing pancreatic malignancies, detecting ACC on EUS-FNA cytology is a challenge due to its rarity. In this study, we aimed to evaluate the cytomorphologic features of ACC on LBC, and also to identify cytological features that help distinguish ACC from PanNENs and SPNs, which are more commonly encountered in everyday practice.
Although CS was the main preparation method for EUS-FNA specimens from the pancreas, many institutions are currently transitioning to LBC for reasons including less background blood or crushing/drying artifacts, resulting in superior sensitivity, accuracy, and negative predictive value [14,15].
When we compared LBC and CS preparations of ACC cases, ACCs commonly presented with hypercellular smears (100%) with frequent single cells and naked nuclei in the background (75.0%) on LBC slides. Prominent nucleoli and macronucleoli could be observed in all LBC cases, while they were less discernible on CS preparations. Similarly to a previous study by Chun et al. [6], we also observed more frequent bloody backgrounds in CS (86.0%) than in LBC (13.0%). The bloody background on CS is more likely to obscure the characteristic cytological features of ACC, such as the acinar architecture and prominent nucleoli, leading to a different diagnostic interpretation. Indeed, in our cohort, the two ACC cases that were originally interpreted as adenocarcinoma were CS cases. Other background features that were often observed in ACCs, including necrotic, apoptotic debris, and nuclear tangles, were similarly present on both CS and LBC slides.
Next, we compared the LBC features of ACC, SPN, and PanNEN. Presence of necrosis and apoptotic debris in the background were significant cytological features suggestive of ACC, and nuclear tangles were only observed in ACC. As for the tumor cell architecture, frequent 3-dimensional pseudopapillary structures were only seen in SPNs. In contrast, the presence of frequent acinar structures was significantly more common in ACCs. As observed in one case in our cohort, acinar-like arrangements may be seen in SPNs; however, the characteristic pseudopapillary structures point to a diagnosis of SPN rather than ACC [16,17]. Acinar structures were not observed in PanNENs.
Cytoplasmic granularity was seen in ACC, SPN, and PanNEN; however, the coarse granularity due to zymogen granules could be better appreciated in ACCs due to the more abundant cytoplasm [16,18,19]. Although nucleoli were observed in all three tumor types, the presence of macronucleoli was unique to ACC, as previously reported [3,20]. The chromatin was irregular for all cases of ACC and PanNEN, while fine chromatin was a characteristic of SPN. In sum, we found that a necrotic background with apoptotic debris, nuclear tangles, acinar structure, presence of macronucleoli and irregular chromatin on LBC were significantly associated with ACC.
Other differential diagnoses to consider include benign acinar cells and other neoplastic lesions including intraductal oncocytic papillary neoplasms or mixed acinar-ductal/neuroendocrine neoplasms. There are situations where reactive acini in pancreatitis could be misinterpreted as acinar cell neoplasia on cytology specimens [12,21]. HooKim et al. [5] demonstrated that the presence of syncytial clusters, prominent cherry red nucleoli and necrosis could be useful clues for the distinction between reactive pancreatic acini and ACC. ACC may show overlapping cytologic features with intraductal oncocytic papillary neoplasm; however, ACC is more mitotically active, and demonstrates a necrotic background [22]. Another differential diagnosis to consider is pancreatic neuroendocrine carcinoma (PanNEC), especially large-cell PanNEC, which is characterized by large cells with relatively abundant eosinophilic cytoplasm, prominent nucleoli, and often a necrotic background. These features overlap with those of ACC, and a definitive diagnosis may not be possible based on cytology without the help of immunocytochemical stains. Lastly, some mixed acinar-ductal or mixed acinar-neuroendocrine neoplasms are often difficult to diagnose accurately on cytology due to limitations such as sampling errors [11,13].
In conclusion, we found that ACC had characteristic cytological features that could be observed on LBC preparations, such as high cellularity, necrotic/apoptotic background, nuclear tangles, acinar arrangement of cells, and macronucleoli. These findings also help distinguish ACC from PanNEN and SPN on LBC. It is important to be familiar with these features, as an accurate diagnosis on EUS-FNA cytology would have impact on the management of the patient.
Notes
Ethics Statement
This study was approved by the Institutional Review Board of Seoul National University Hospital (IRB No. H-2402-011-1506), and informed consent was waived due to the retrospective nature of the study.
Availability of Data and Material
The datasets generated or analyzed during the study are available from the corresponding author on reasonable request.
Code Availability
Not applicable.
Author Contributions
Conceptualization: HK. Data curation: HK, MK. Formal analysis: MK. Funding acquisition: HK. Investigation: HK, MK. Methodology: MK, SMH. Resources: MK, HK, KL, SMH. Supervision: HK, SMH. Visualization: MK. Writing—original draft: MK. Writing—review & editing: MK, HK, KL, SMH. Approval of final manuscript: all authors.
Conflicts of Interest
H.K., a contributing editor of the Journal of Pathology and Translational Medicine, was not involved in the editorial evaluation or decision to publish this article. All remaining authors have declared no conflicts of interest.
Funding Statement
No funding to declare.