Colorectal cancer with a germline BRCA1 variant inherited paternally: a case report
Article information
Abstract
BRCA genes have well-known associations with breast and ovarian cancers. However, variations in the BRCA gene, especially germline variations, have also been reported in colorectal cancer (CRC). We present the case of a rectal cancer with a germline BRCA1 variation inherited from the paternal side. A 39-year-old male was admitted with rectal cancer. The patient underwent surgical resection and the pathologic diagnosis was adenocarcinoma. Next-generation sequencing was performed and a BRCA1 variant was detected. Reviewing the public database and considering the young age of the patient, the variant was suggested to be germline. The patient’s father had had prostate cancer and next-generation sequencing testing revealed an identical BRCA1 variant. In the BRCA cancer group, there is relatively little attention paid to male cancers. The accumulation of male CRC cases linked to BRCA variations may help clarify the potential pathological relationship between the two.
Colorectal cancer (CRC) is the third-most common cancer and a second-leading cause of cancer-related death worldwide [1]. Sporadic CRC is the most common form of CRC, accounting for 65% to 85% of cases [2]. However, the hereditary form also comprises a significant part of in CRCs. Well-known hereditary diseases that increase the incidence of CRC include Lynch syndrome, which consists of 3% to 8% of CRC cases, familial adenomatous polyposis, which accounts for approximately 1%, and MUTYH-associated polyposis, which comprises <1% of cases [2].
The BRCA1 and BRCA2 tumor suppressor genes are involved in multiple cellular functions, including DNA repair, cell cycle regulation, and transcription [3]. Associations between germline variations in BRCA1 and BRCA2 genes and various types of cancer including breast, ovarian, pancreas, and prostate cancers have been reported since the discovery of the genes. Recently, as sequencing for CRCs has risen with the introduction of next-generation sequencing (NGS), reports that BRCA gene variation may play a role in the development of CRC are increasing [4]. However, the clinical significance of BRCA gene variation in the development of CRCs remains unclear [4-7]. To date, there have been many case reports of germline BRCA1 variation in CRC. However, most reports have focused on women, and cases involving men primarily involved reported variations inherited from their maternal side; reports of BRCA1 inherited from the paternal line in CRC patients are very rare. Herein, we present the case of a patient that developed rectal cancer who harbored a pathogenic germline alteration in the BRCA1 gene inherited from the paternal side.
CASE REPORT
A 39-year-old male was admitted to our hospital with a three-to four-month history of painless bloody stools. He underwent endoscopic evaluation of the gastrointestinal tract. Colonoscopy revealed an approximately 4 cm mass on the rectum, which was biopsied (Fig. 1A). The rectal mass was diagnosed as adenocarcinoma. Abdominal computed tomography showed a 3.8×2.6-cm-sized rectal mass infiltrating the mesorectal fat (cT3) with adjacent regional lymph nodes suspicious for nodal metastasis (cN1) (Fig. 1B). Chest computed tomography did not show any evidence of metastasis. Before surgery, the patient received neoadjuvant chemoradiation therapy. After chemoradiotherapy, the patient underwent surgical resection and pathologic diagnosis was moderately differentiated adenocarcinoma with metastasis to one regional lymph node (ypT2N1a). The patient’s tumor regression grade was 1 (moderate response) according to American Joint Committee on Cancer (AJCC) tumor regression staging [8]. Immunohistochemistry for mismatch repair protein including MLH1, MSH2, MSH6, and PMS2 showed intact nuclear expression.
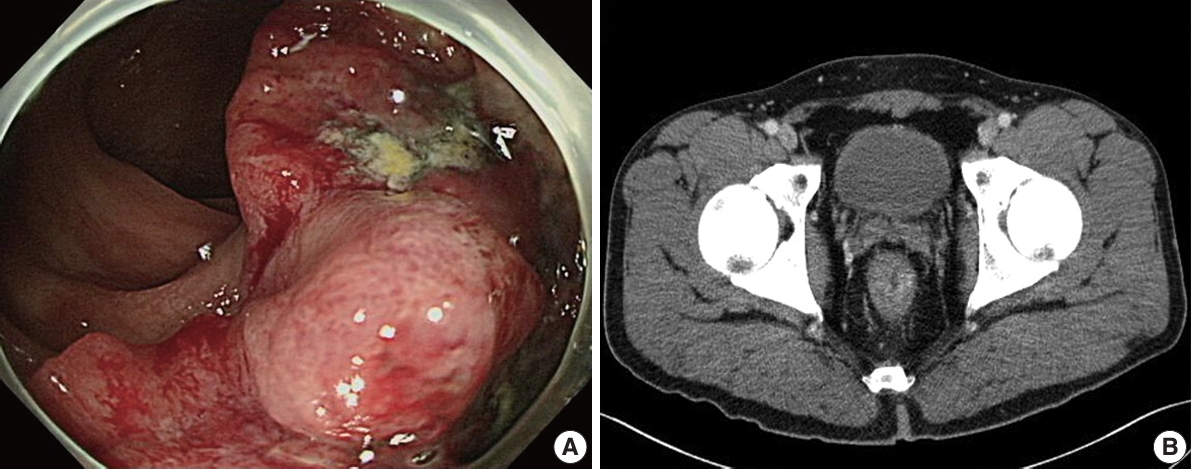
Colonoscopy and abdominal computed tomography findings. (A) Endoscopy of the lower gastrointestinal tract revealed a mass in the rectum. (B) Computed tomography showed an approximately 4-cm thickening 3 cm from the anal verge.
The patient reported a family history of cancer; his father had developed prostate cancer previously. However, the patient’s mother had no history of cancer. Considering the patient’s age and family history of cancer, genetic analysis of the tumor was recommended. NGS was performed with an Oncomine Comprehensive Assay v1 panel (OCAv1, Thermo Fisher Scientific, Waltham, MA, USA). NGS revealed variation in the BRCA1 gene (c.2713C>T, p.Gln905*) (Fig. 2A). The variant allele frequency was 53.9%. This variant is reported in public databases including Clinvar as a pathologic germline variant mainly associated with familial breast and ovarian cancer. In addition to the BRCA1 variation, NGS of the tumor tissue revealed mutations in TP53 (c.524G>A, p.Arg175His) and PPP2R1A (c.547C>T, p.Arg183Trp). To confirm the BRCA1 variant to be of germline origin, the patient’s normal tissue was sequenced through NGS and an identical BRCA1 variation (c.2713C>T, p.Gln905*) was detected (Fig. 2B). The patient’s mother had no history of cancer. The patient’s father was diagnosed with prostate cancer at the same hospital 2 years before the patient was diagnosed with CRC. At that time, no genetic study was conducted on the prostate cancer tissue. We obtained prostate cancer tissue from archived FFPE samples and sequenced the tissue using the same NGS panel, and an identical BRCA1 variation, c.2713C>T, p.Gln905*, was detected. Therefore, we concluded that the patient’s BRCA1 variation was inherited from the paternal side.
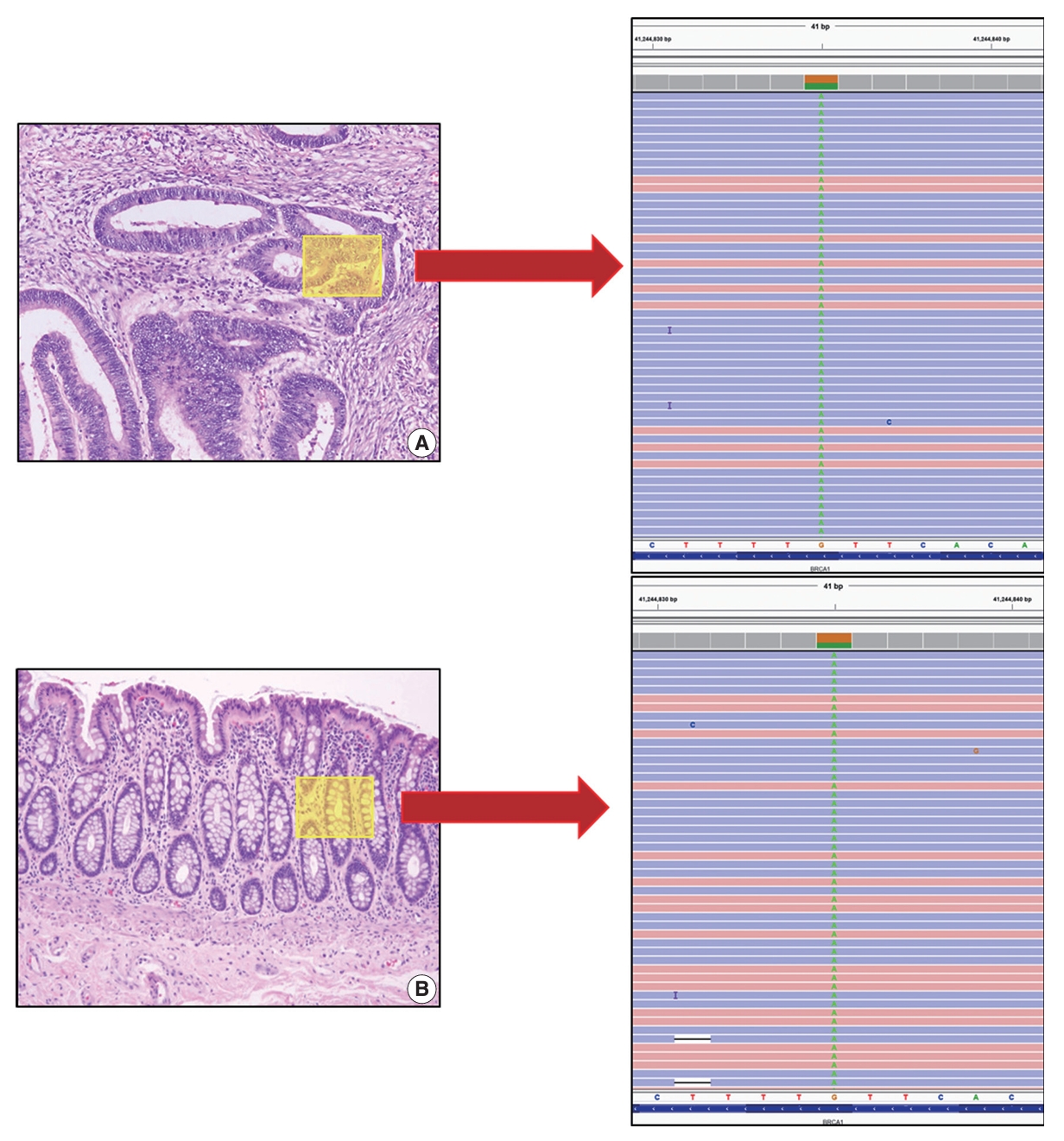
Next-generation sequencing was performed on the rectal adenocarcinoma tissue (A) and normal colonic tissue (B). The results of both revealed BRCA1 (c.2713 C>T, p.Gln905*) variation.
Alterations in the BRCA gene are closely associated with the development of breast and ovarian cancers. The patient had two daughters, and we recommended genetic studies of BRCA1 in the patient’s two daughters at a later date (after age 18).
The patient subsequently completed adjuvant treatment with eight cycles of 5-fluorouracil (5-FU)– and oxaliplatin-based chemotherapy. After 2 years of follow-up, the patient is alive without recurrence or metastasis.
DISCUSSION
The role of BRCA genes in repairing DNA double-strand breaks by homologous recombination is well established [3]. BRCA variants contribute to the development of many cancers [3]. The cancers most commonly associated with BRCA mutation are breast and ovarian cancer [9]. Germline variants in BRCA genes lead to increased lifetime risk of up to 85% for breast cancer and 40% for ovarian cancer [9]. Also, BRCA variants are associated with prostate, melanoma, pancreas, and stomach cancer [10]. However, studies investigating the relationship between BRCA and development of CRCs have shown conflicting results [5-7]. Research suggests that BRCA1 and BRCA2 mutations occur in less than 1% of CRC cases [11,12]. A comprehensive study utilizing The Cancer Genome Atlas data found that the prevalence of BRCA1 and BRCA2 mutations in CRC patients was approximately 0.1%–0.5% [11,12]. Additionally, germline BRCA variation in CRC have mostly focused on women or were inherited from the maternal line. To the best of our knowledge, this is the first report of germline BRCA variation in CRC that was inherited from the paternal side.
Studies investigating the effects of germline variations in BRCA on the occurrence of cancers other than breast and ovarian cancers are being actively conducted [10,13]. Some reports indicated that CRC was significantly elevated in BRCA1 carriers [13]. However, there have been other studies showing that BRCA1 mutations do not affect the development of CRC [7]. Additionally, many of the studies regarding the development of CRC and BRCA mutations only included women, especially women with a history of breast or ovarian cancer harboring germline BRCA mutations [4]. There is relatively little interest in men with BRCA mutations, and this may lead to delayed detection of cancer in male patients.
In a previous study, the risk of early-onset CRC was significantly increased in BRCA1 carriers and the cutoff used was <50 years of age [4]. In a report related to surveillance of male BRCA carriers, it was suggested that cancer monitoring should be started at the age of 40 [14]. A recent case report described early-onset CRC with a germline BRCA1 mutation in a 33-year-old man [15]. In our case, the patient was 39 years old. When CRC occurs in a young patient, efforts must be made to distinguish whether it is a sporadic or hereditary cancer. Although BRCA mutations are not frequent, it may be necessary to investigate the possibility of BRCA variations in cases of CRC not involving Lynch syndrome, familial adenomatous polyposis, or MUTYH-associated polyposis.
Alterations in BRCA genes contribute to the development of prostate cancer [16]. A recent study showed that among 620 prostate cancer patients, 6.4% had germline BRCA variations [17]. Family history is an important risk factor for prostate cancer. Therefore, our patient may be at higher risk for prostate cancer than men without a BRCA mutation, so regular prostate cancer screening using serum prostate-specific antigen levels is recommended.
Male patients with BRCA mutations are at increased risk of several cancers, including breast, prostate, pancreatic, and CRC [18]. Early and regular screening is essential for early detection and improved outcomes. The National Comprehensive Cancer Network provides guidelines for genetic testing and early cancer screening for individuals with BRCA mutations [18]. These guidelines recommend that male BRCA mutation carriers undergo regular screening for prostate cancer starting at age 40, and screen for breast cancer through regular self-exams and clinical exams starting at age 35 [18]. In CRC, although the association with BRCA mutations is less pronounced, some studies suggest that BRCA mutation carrier, may benefit from earlier and more frequent screening, especially those with a family history of CRC [19].
BRCA-mutated cancers are sensitive to platinum-based therapy [20]. The effectiveness of platinum-based therapy in BRCA-mutant cancers are well established in breast and ovarian cancers. Although studies are limited in BRCA-mutant CRCs, one report described a case of rectal cancer in a patient with a germline BRCA1 variant [17]. In that report, the patient showed a complete response to neoadjuvant chemoradiation, and a platinum-based agent was included in the chemotherapeutic regimen [15]. The authors added a platinum agent in the neoadjuvant regimen based on the detection of the germline BRCA1 variant and hypothesized that adding a platinum agent might have been responsible for excellent response [15]. Our patient showed a moderate response on AJCC tumor regression grading after standard neoadjuvant chemoradiation, which included radiation (5,040 cGy) and capecitabine. We did not add a platinum agent to the neoadjuvant therapy regimen because the mutation status of the rectal cancer was not available at that time. After surgery, the patient received eight cycles of 5-FU– and oxaliplatin-based chemotherapy. The patient is alive without recurrence or metastasis.
To confirm the functional significance of the BRCA1 pathogenic variant, it is crucial to have information on its zygosity or the presence of loss of heterozygosity (LOH) in tumor tissue. If homologous recombination deficiency (HRD) is confirmed in the CRC tissue, it would strongly suggest an association with BRCA. In this case, although a BRCA1 mutation was identified, HRD and LOH statuses were not assessable at the time of examination. Additionally, a TP53 mutation was observed, complicating the determination of whether the BRCA1 mutation acted as a driver mutation in CRC carcinogenesis. Given these findings, it was not possible to definitively conclude that the BRCA1 mutation was a functional driver mutation for CRC. These limitations should be considered when interpreting the results of this study, and further research is warranted.
In conclusion, we presented a case of CRC in a 39-year-old patient with a germline BRCA1 variant inherited from the paternal line. Although our observation has limitations because it is a single case report, testing for germline BRCA mutations should be considered in relatively young CRC patients, especially those who have a family history of BRCA-related cancer. The accumulation of male CRC cases linked to BRCA variations can help clarify the potential pathological relationship between the two.
Notes
Ethics Statement
This study was approved by the Institutional Review Board of the Jeonbuk National University Hospital with a waiver of informed consent (IRB No. 2021-12-027).
Availability of Data and Material
The datasets generated or analyzed during the study are available from the corresponding author on reasonable request.
Code Availability
Not applicable.
Author Contributions
Conceptualization: MJC. Data curation: KMK. Formal analysis: ARA, KMK. Investigation: KMK. Methodology: MJC, ARA. Supervision: MJC, MRL. Validation: ARA, KMK, MJC. Writing—original draft: KMK. Writing—review & editing: MRL, MJC. Approval of final manuscript: all authors.
Conflicts of Interest
The authors declare that they have no potential conflicts of interest.
Funding Statement
This paper was supported by Fund of Biomedical Research Institute, Jeonbuk National University Hospital.
