International Academy of Cytology standardized reporting of breast fine-needle aspiration cytology with cyto-histopathological correlation of breast carcinoma
Article information
Abstract
Background
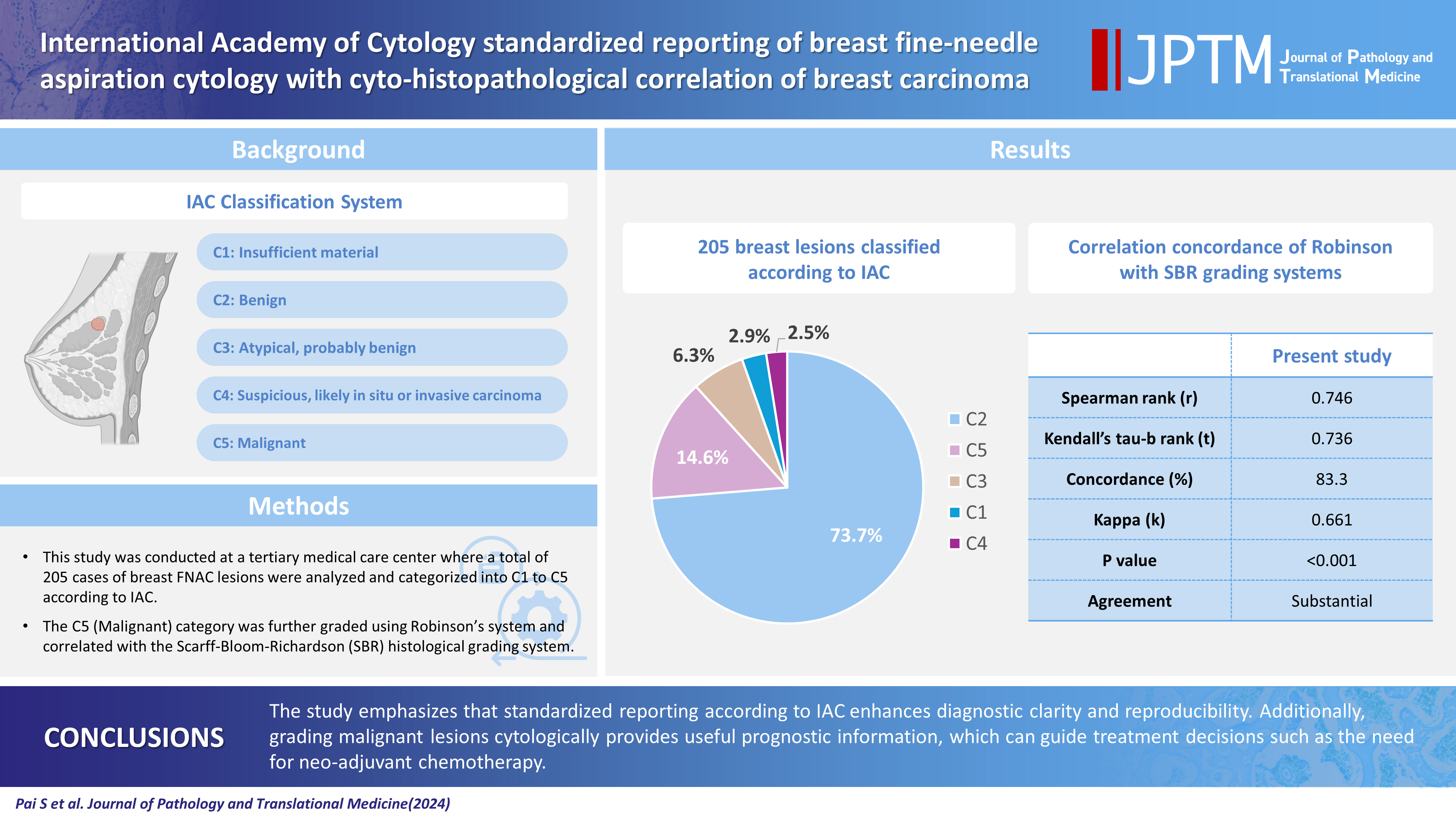
The International Academy of Cytology (IAC) has developed a standardized approach for reporting the findings of breast fine-needle aspiration cytology (FNAC). Accordingly, there are five chief categories of breast lesions, C1 (insufficient material), C2 (benign), C3 (atypical), C4 (suspicious), and C5 (malignant). The prognostication and management of breast carcinoma can be performed readily on the basis of this classification system. The aim of this study was to classify various breast lesions into one of the above-named categories and to further grade the C5 lesions specifically using the Robinson system. The latter grades were then correlated with modified Scarff-Bloom-Richardson (SBR) grades.
Methods
This retrospective study was undertaken in the pathology department of a hospital located in the urban part of the city of Bangalore. All FNAC procedures performed on breast lumps spanning the year 2020 were included in the study.
Results
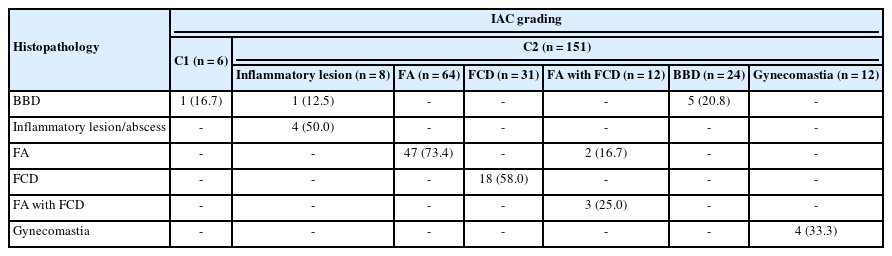
A total of 205 breast lesions was classified according to the IAC guidelines into C1 (6 cases, 2.9%), C2 (151 cases, 73.7%), C3 (13 cases, 6.3%), C4 (5 cases, 2.5%), and C5 (30 cases, 14.6%) groups. The C5 cases were further graded using Robinson’s system. The latter showed a significant correlation with the SBR system (concordance=83.3%, Spearman correlation=0.746, Kendall’s tau-b=0.736, kappa=0.661, standard error=0.095, p≤.001).
Conclusions
A standardized approach for FNAC reporting of breast lesions, as advocated for by the IAC, improves the quality and clarity of the reports and assures diagnostic reproducibility on a global scale. Further, the cytological grading of C5 lesions provides reliable cyto-prognostic scores that can help assess a tumor’s aggressiveness and predict its histological grade.
The prevalent perception of breast cancer as the most common kind of cancer affecting predominantly the Western hemisphere is no longer true. For instance, breast cancer was recently reported to have overtaken cervical cancer in India to become the most common cancer. According to the Indian Council of Medical Research, 150,000 new cases of breast cancer were reported in the year 2016 [1]. This surging new trend has directed the resolve of the national health planning sector toward identifying the best-available diagnostic tools for early-stage cancer detection. Mass deployment of such a tool would help to ensure better treatment outcomes marked by increased life expectancy of the survivors. By a majority consensus, fine-needle aspiration cytology (FNAC) was identified as one such ideal technique. The International Academy of Cytology (IAC) has formulated a process to ensure a standardized and comprehensive approach to FNAC reporting. Accordingly, they have categorized breast lesions into five categories, C1–C5 (C-code). At the inaugural Yokohama International Congress, attended by breast cancer specialists, there was a discussion on the use of a three- or a five-stage coding system. Congress attendees ultimately reached a consensus to employ a five-stage system: category 1, insufficient material; category 2, benign; category 3, atypical, probably benign; category 4, suspicious, probably in situ or invasive carcinoma; category 5, malignant.
Since the proposal of the aforementioned coding system, it has been widely employed on a worldwide scale. However, the respective definitions for categories 3 and 4 have recently come under scrutiny, as there is a recognized need for further debate on the grey area of atypia [2]. The IAC has attempted to refine these definitions by outlining specific criteria or scenarios wherein atypia can be deemed an appropriate diagnosis, which include the presence of epithelial hyperplasia with marked dispersal of columnar cells and minimal nuclear atypia, where the differential diagnosis is epithelial hyperplasia or low-grade intraductal carcinoma; the presence of intraductal papillomas with marked dispersal and diagnostic stellate papillary fragments, where the differential diagnosis is low-grade intraductal carcinoma; the presence of epithelial hyperplasia with a complex cribriform or micropapillary pattern, where the differential diagnosis is low-grade intraductal carcinoma; the presence of stromal hypercellularity without necrosis or nuclear atypia in a case of otherwise typical fibroadenoma, with consideration of the possibility of a low-grade phyllodes tumor; and the presence of smears with scant cellularity but minute epithelial fragments and single cells exhibiting eccentric cytoplasm, where the differential diagnosis is lobular carcinoma in situ or lobular carcinoma.
The IAC has established a checklist for FNAC of breast lesions, where an analytical approach based on cytological diagnostic criteria and pattern recognition can be used by cytopathologists for generating reports [2].
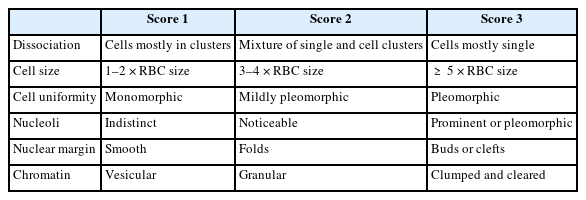
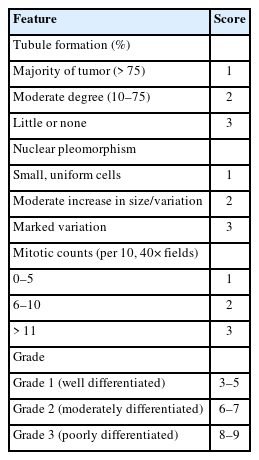
Histological grading of breast carcinoma employing the Elston-Ellis modification of the Scarff-Bloom-Richardson (SBR) grading system is a well-known and accepted approach with good correlation prognostically [3]. However, significant ambiguity persists around cytological grades. While many systems have been proposed to grade breast cancers preoperatively, the grading system of Robinson et al. best correlates with the SBR system [4-11].
In this current age of neo-adjuvant chemotherapy, it is highly recommended that the FNAC report incorporates grading of breast carcinoma. This would not only assist in the prognostication of breast cancer, but also be useful in cases that reject surgeries, cases with locally advanced disease, and cases of elderly patients with other co-morbidities [12,13].
Hence, the aim of the present study was to classify lesions of breast cancer diagnosed by FNAC according to the IAC scheme (C1–C5) with additional grading of malignant breast lesions (C5) using Robinson’s system and to finally correlate the cytological grading with the standard Elston-Ellis modification of the SBR system.
MATERIALS AND METHODS
The current retrospective study was undertaken in the Department of Pathology at a tertiary medical care center in Bangalore city. A total of 205 patients who underwent an FNAC procedure for breast lumps between January 1, 2020, and December 31, 2020, were included in the study. Any cases subjected to neo-adjuvant chemotherapy were excluded. The FNAC technique was performed using 10-cc syringes with a 22–23-gauge needle under aseptic conditions. The samples obtained were smeared on glass slides and fixed with 95% ethyl alcohol. Staining was performed using hematoxylin and eosin and Leishman’s method. IAC standardized reporting was used to classify all breast lesions into distinct C1–C5 categories. Further, the lesions that belonged to the C5 category were cytologically graded using Robinson’s system.
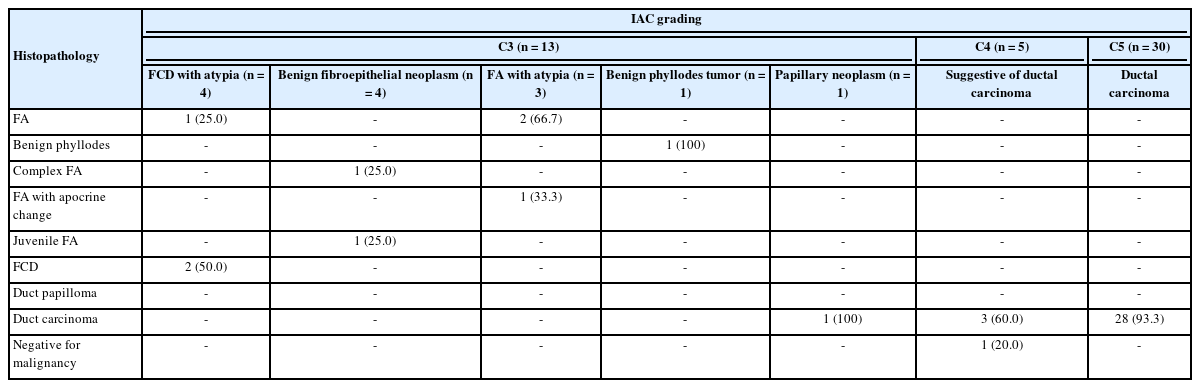
The subsequent mastectomy specimens of breast carcinoma, received in the histopathology department, were fixed in 10% formalin. These specimens were grossly and aptly sampled after adequate fixation. The tissue samples were processed, and slides were prepared. Staining was performed using hematoxylin and eosin stain. Histological typing and further grading were performed using the gold-standard Elston-Ellis modification of the SBR grading system. Finally, cytological and histological grades were correlated for all the breast carcinoma cases (Tables 1, 2) [3,4].
Statistical analysis, including descriptive analysis and contingency table analysis (cross-tabulation procedure), was performed using SPSS software ver. 20 (IBM Corp., Armonk, NY, USA).
RESULTS
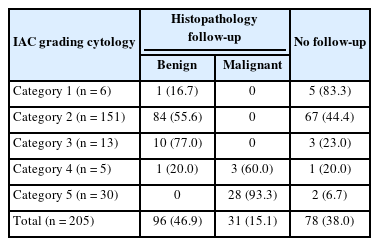
In the present study, 205 patients aged 20–60 years underwent FNAC for breast masses. According to the IAC standardized reporting, they could be graded with a C-code (C1–C5). Follow-up data were available for 127 of the cases, among which 96 were benign and 31 were malignant (Table 3).
Category 1: Insufficient material
Smears that do not show epithelial cells are labeled as insufficient or inadequate collections. According to the MD Anderson Cancer Center Group’s proposal, the presence of 4–6 well-visualized groups ≥10 cells or flat sheets is considered adequate [14].
In the current study, the C1 category comprised six cases (2.9%), one of which was diagnosed as a case of benign breast disease on follow-up (Table 4).
Category 2: Benign
Category 2 comprised lesions showing benign epithelial clusters with no atypical or malignant features. High cellularity is rare in these cases, and cells present a low nucleocytoplasmic ratio, fine chromatin, no pleomorphism, and a smooth nuclear membrane. Fatty fragments are common, along with bare nuclei. Few cases show apocrine cells or histiocytes.
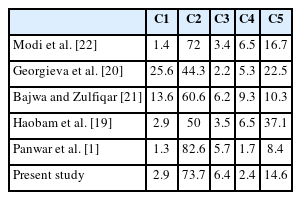
In the present study, most lesions belonged to the C2 category (151 cases, 73.7%). Among these 151 lesions, 64 (42.4%) were fibroadenomas, 31 (20.5%) were fibrocystic diseases, 24 (15.9%) were benign breast diseases, 12 (8.0%) were fibroadenoma with fibrocystic disease and gynecomastia, and 8 (5.3%) were inflammatory breast lesions (Fig. 1).
C2 (benign category). (A, B) Fibroadenoma; note the branching sheets of cohesive cells giving the antler horn pattern (B, Leishmans). (C, D) Fibroadenoma with epithelial hyperplasia.
In the C2 category, follow-up data were available for 84 cases, including 47 cases of fibroadenomas, 18 cases of fibrocystic diseases, five cases of fibroadenomas with fibrocystic disease, four cases of gynecomastia, five cases of inflammatory breast lesions, and five cases of benign breast diseases (Table 4).
Category 3: Atypical, probably benign
Findings of category 3 lesions are similar to those in the benign category but with slight crowding, greater cellularity, pleomorphism, three-dimensional grouping, and nuclear enlargement.
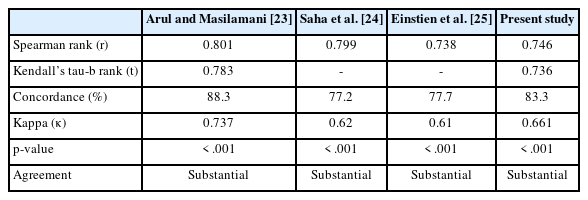
C3 lesions in our study were present in 13 cases (6.4%), including four (30.8%) each of fibrocystic disease with atypia and benign fibroepithelial neoplasm, three cases (23%) of fibroadenoma with atypia, and one case (7.7%) each of benign phyllodes tumor and papillary neoplasm (Fig. 2).
C3 (atypical category). (A, B) Benign phyllodes tumor. Note the abundance of stromal fragments. The inset shows a high-power view (B, Leishmans).
Follow-up data were available for 10 C3 cases, including three cases each of fibrocystic disease with atypia and fibroadenoma with atypia, two cases of benign fibroepithelial neoplasms, and one case each of benign phyllodes and a papillary neoplasm (Table 5).
Category 4: Suspicious, probably in situ or invasive carcinoma
Category 4 lesions exhibit highly atypical findings that remain insufficient to label them malignant. Cases in this category include those with poorly preserved or hypocellular smears, those with focally atypical cells in a benign background, and those with an insufficient degree of atypia to classify C5 but where the atypia is greater than that of C3.
In the current study, C4 lesions suspicious for malignancy accounted for five cases (2.4%). On follow-up, three cases were confirmed to be ductal carcinoma; however, one case was negative for malignancy (Table 5).
Category 5: Malignant
These lesions show high cellularity. Cells are seen in singles or loose clusters, and malignant features such as hyperchromasia, high nucleocytoplasmic ratio, irregular nuclear contour, and conspicuous nucleoli are noted. Some cases show necrotic debris in the background.
In the present study, C5 lesions were the second most common entity, found in 30 cases (14.6%).
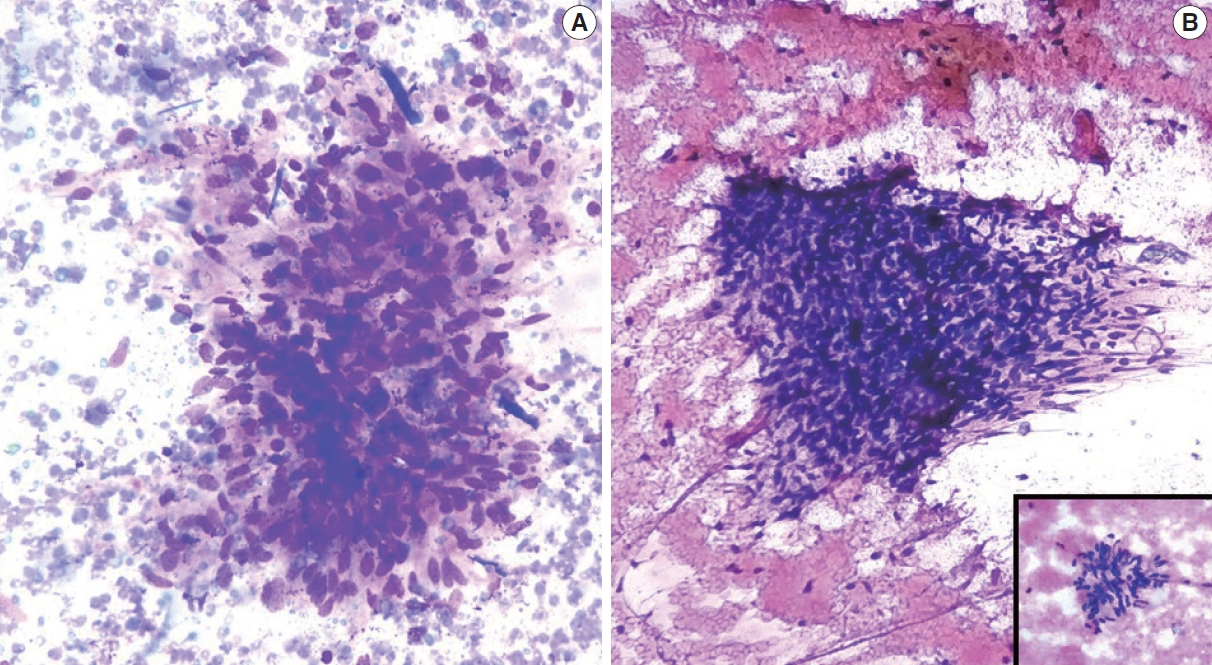
Follow-up was available for only 20 C5 cases, which were all confirmed to be ductal carcinoma (Table 5, Fig. 3).
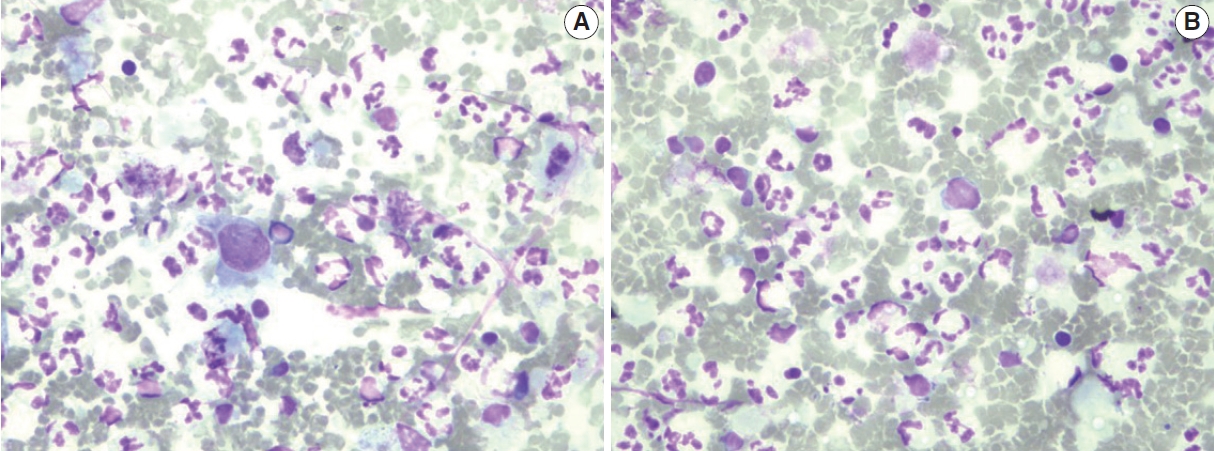
C4 (suspicious for malignancy category). (A, B) Occasional malignant cells can be seen in the background of inflammatory cells.
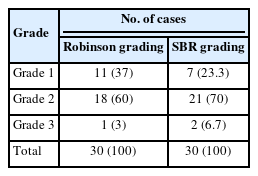
All C5 cases underwent cytological grading using Robinson’s system [4], and histopathological grading was completed using the Elston-Ellis modification of the SBR grading system [3].
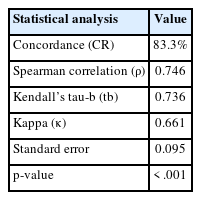
In the present study, the Robinson grades showed very good concordance (83.3%) with the modified (Elston-Ellis) SBR grades, with high correlation (0.746), high Kendall’s tau-b (0.736), high kappa value (0.661), substantial agreement, and a significant p-value (<.001) (Tables 6, 7, Fig. 4).
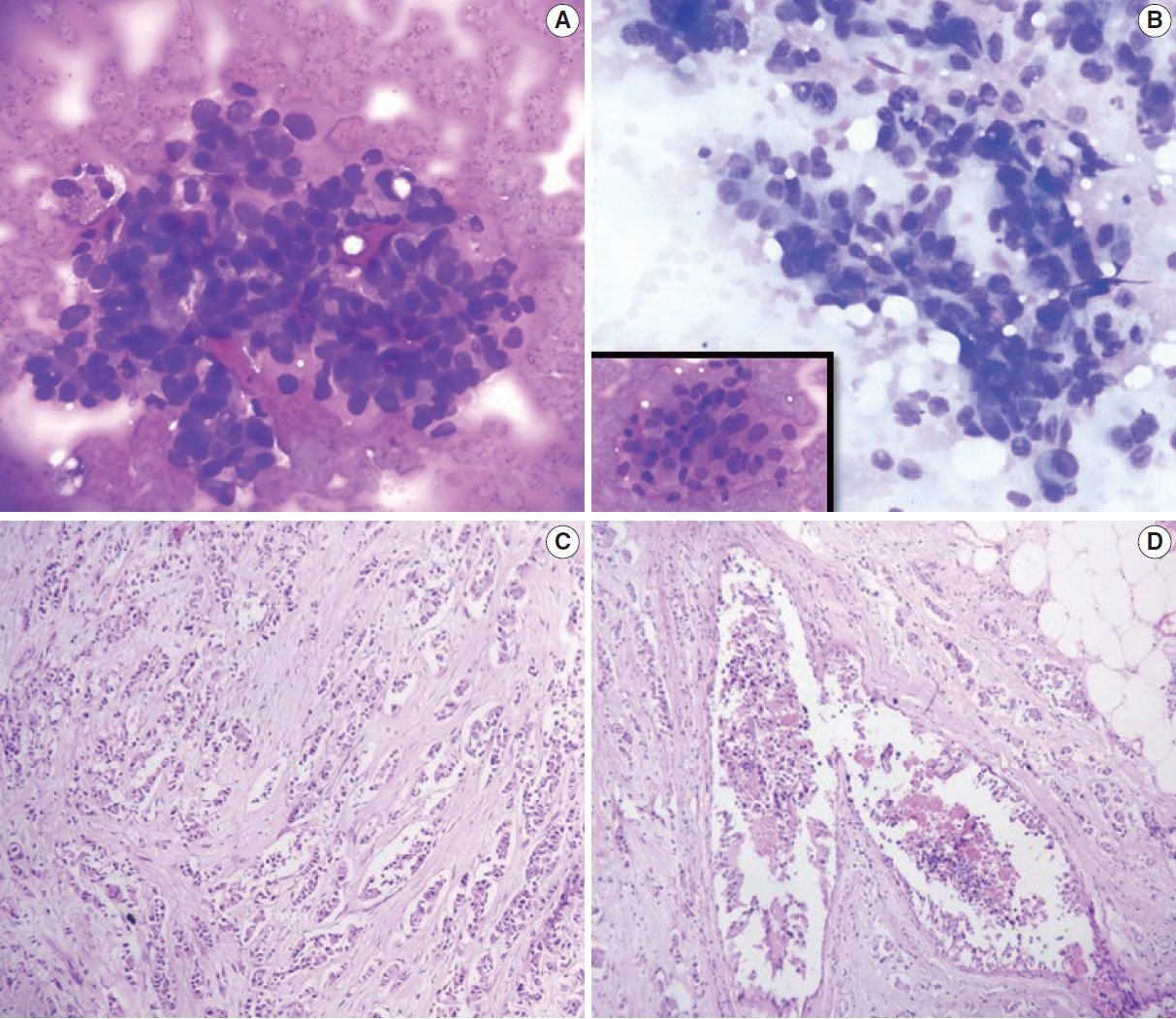
C5 (malignant category). Invasive ductal carcinoma. (A, B) Cytological images; note the discohesive cluster of malignant cells with a high nucleocytoplasmic ratio, irregular nuclear membrane, and clumped chromatin. The inset shows a high-power view. (C, D) Histopathological images. Note the infiltrative cords, small nests, and occasional tubules of tumor cells within a prominent desmoplastic stroma.
DISCUSSION
Diagnosing breast cancers at an early stage is imperative for favorable treatment outcomes, but cases often go undiagnosed for socio-economic reasons. Hence, it is of paramount importance that an easy, cost-effective, and reliable investigation like FNAC be performed in such circumstances [15]. FNAC is a rapid, accurate, and relatively painless procedure [16]; however, translation of cytological patterns into histopathological patterns can be difficult, hindering diagnosis [17]. In such a scenario, structured reporting using IAC guidelines will not only help improve the quality and accuracy of the reports, but also ensure reproducibility of the findings on a global scale [2].
Category 1
Cases in the C1 category include those with an inadequate/insufficient sample, such as cases marked by hypocellular smears, errors in spreading/staining, excessive blood, crushing artifacts, degenerated cells, and poor fixation. However, cases with cysts, abscess, fat necrosis, or intra-mammary lymph nodes are not considered C1. The risk for malignancy among C1 cases is 4.8% [18]. As shown in Table 8, our study documented a C1 prevalence of 2.9%, which was identical to that reported by Haobam et al. [19]. Meanwhile, Georgieva et al. [20] and Bajwa and Zulfiqar [21] reported much higher frequencies of C1 cases, which could be explained partly by the use of faulty techniques and inadequate expertise [22].
Category 2
Cases in the C2 category are considered benign and include instances of fibroadenoma, fat necrosis, abscess, granulomatous mastitis, and other benign entities such as intra-mammary lymph nodes. Cytology smears in these cases will demonstrate a regular ductal epithelium, cysts, or fibrofatty fragments depending on the etiology. The risk for malignancy is 1.4% [18].
Most of the cases in our study were C2 cases (73.7%), concurring with rates in other studies (Table 8). Among the C2 cases, fibroadenomas were most common (42%), as reported by Modi et al. [22], Panwar et al. [1], and Bajwa and Zulfiqar [21]. The second most common C2 lesions were those indicating fibrocystic disease (21%), as in Bajwa and Zulfiqar [21].
Category 3
Cases in the C3 category are atypical but probably benign and include papillary lesions and suspected phyllodes tumors. Cytology smears in such instances may show pleomorphism, increased cellularity, and loss of cohesion. The risk of malignancy is 13% [18]. No definitive surgery is recommended for cases in this category. The prevalence of C3 cases was 6.4% in the present study, similar to reports by both Bajwa and Zulfiqar [21] and Panwar et al. [1]. Meanwhile, Georgieva et al. [20] encountered a much lower frequency of C3 lesions (Table 8).
Category 4
Category 4 cases are suspicious for malignancy. Cytology findings may show scant/poor preservation of cells, along with occasional malignant cells or some features of malignancy. The overall risk of malignancy is 97.1% [18]. However, no definitive surgery is recommended for this category. Panwar et al. [1] reported very few cases (1.7%) of C4 lesions, which concurred with observations in our study. However, other studies have reported higher frequencies of C4 cases (Table 8).
Category 5
Category 5 is the malignant category. Here, cytology smears will show overt malignant features like a high nucleo-cytoplasmic ratio, hyperchromasia, irregular nuclear contours, and conspicuous nucleoli. The risk for malignancy is 100% [18]. C5 lesions were the second most common lesions after C2 lesions in our and other studies, which indicates a rising trend in the frequency of breast cancer cases.
In the present study, malignant cases (C5) were accurately identified by FNAC as later confirmed by histopathology. This was also true in Panwar et al. [1]. However, two cases (C5) were lost to follow-up in our study (Table 8).
Robinson’s cytological system was used to grade all C5 lesions. This system is an excellent predictor of the aggressiveness of a tumor; compared to the standard histological grading approach, i.e., the modified SBR system, it exhibits substantial correlation in terms of Spearman’s rank, Kendall’s tau-b rank, concordance, kappa value, and p-value. This finding was reported in other studies like those by Arul and Masilamani [23] and Saha et al. [24] (Table 9) [25-28].
A standardized approach as advocated for by the IAC for FNAC reporting of breast lesions will not only improve the quality and clarity of reports, but also assures their reproducibility, internationally. Furthermore, the cytological grading of C5 lesions provides cyto-prognostic scores that can help assess the aggressiveness of a tumor and predict its histological grade.
Notes
Ethics Statement
All procedures performed in the current study were approved by the central government’s institutional research ethics committee (reference no.: 532/L/11/12/Ethics/ESICMC&PGIMSR/Estt.Vol.IV; Jun 22, 2021) in accordance with the 1964 Declaration of Helsinki and its later amendments. Formal written informed consent was not required, with a waiver granted by the research ethics committee.
Availability of Data and Material
The datasets generated or analyzed during the study are available from the corresponding author on reasonable request.
Code Availability
Not applicable.
Conflicts of Interest
The authors declare that they have no potential conflicts of interest.
Funding Statement
No funding to declare.