Lessons learned from the first 2 years of experience with thyroid core needle biopsy at an Indonesian national referral hospital
Article information
Abstract
Background
Core needle biopsy (CNB) improves diagnostic accuracy by providing precise tissue sampling for histopathological evaluation, overcoming the limitation of inconclusive fine-needle aspiration results. This study evaluated the diagnostic performance of CNB in assessing thyroid nodules, with additional analysis of the benefits of BRAF V600E and RAS Q61R immunohistochemical (IHC) markers.
Methods
This retrospective study enrolled patients with thyroid nodules who underwent CNB at Dr. Cipto Mangunkusumo Hospital, Jakarta, from July 2022 to July 2024. CNB diagnoses were classified using the Korean Thyroid Association Criteria. Diagnostic efficacy was evaluated for neoplastic and malignant lesions, both independently and with BRAF V600E and RAS Q61R IHC. The correlation between nodule size and postoperative diagnosis was also analyzed.
Results
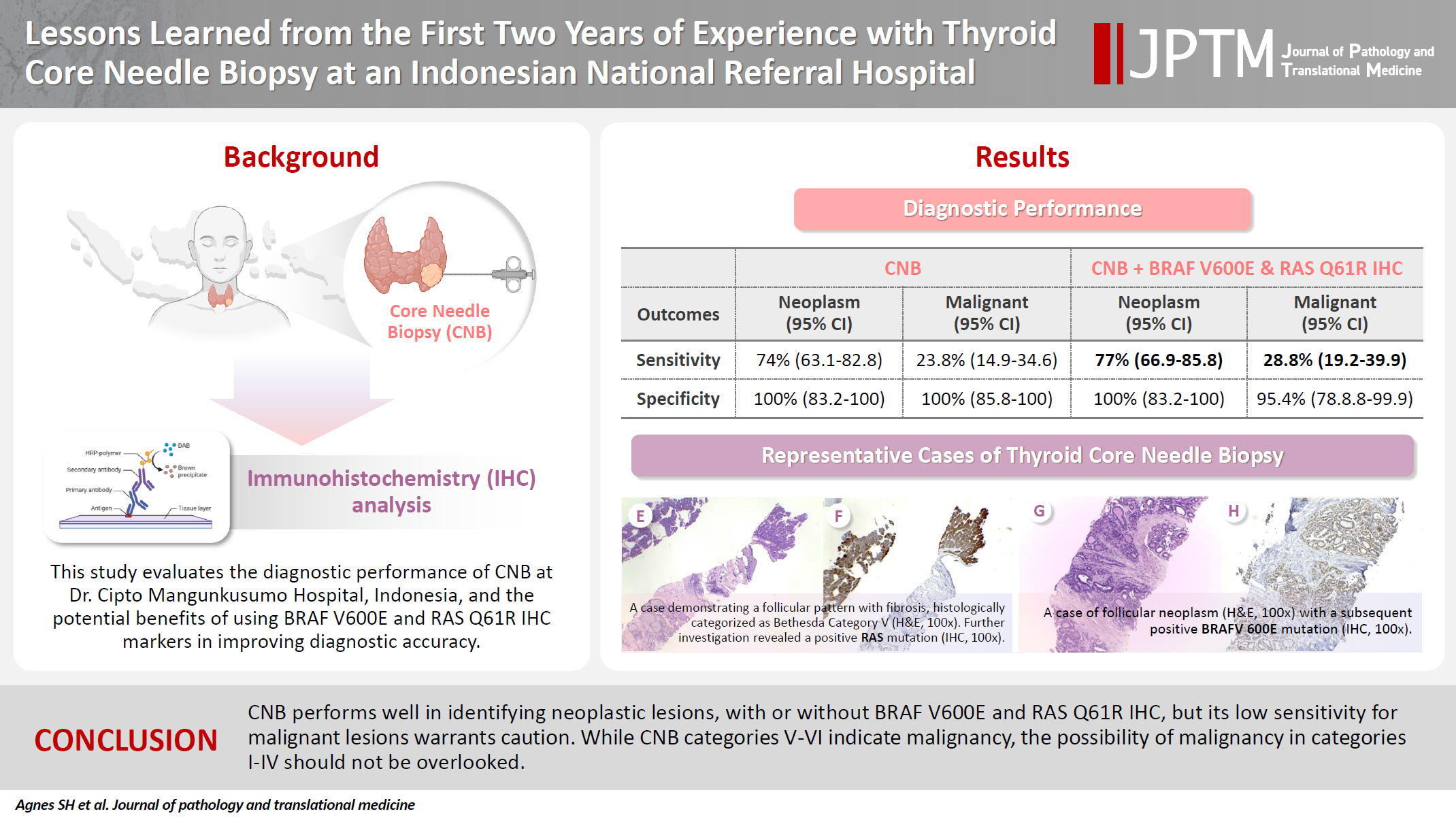
A total of 338 thyroid nodule samples was included, and 52.7% were classified as CNB category II. In the 104 samples with postoperative diagnoses, category IV was the most prevalent (39.4%). CNB demonstrated a sensitivity of 74% and a specificity of 100% for neoplastic lesions and 23.8% sensitivity and 100% specificity for malignant lesions. Combining CNB with BRAF V600E and RAS Q1R IHC increased the sensitivity to 77% for neoplastic lesions and 28.8% for malignant lesions. Larger nodules (>3 cm) were significantly associated with neoplastic (p = .005) and malignant lesions (p = .004).
Conclusions
CNB performs well in identifying neoplastic lesions, with or without BRAF V600E and RAS Q61R IHC, but its low sensitivity for malignant lesions warrants caution. While CNB categories V–VI indicate malignancy, the possibility of malignancy in categories I–IV should not be overlooked.
INTRODUCTION
Ultrasound (US)-guided fine-needle aspiration (FNA) is a reliable and precise technique for the evaluation of thyroid nodules [1]. A significant limitation of FNA is the occurrence of nondiagnostic and indeterminate specimens classified by The Bethesda System for Reporting Thyroid Cytopathology, caused by insufficient cells or collection of bloody material [1]. Two previous studies indicated that the rates of malignancy for nondiagnostic and indeterminate FNA aspirates can reach as high as 10.9% and 60.0%, respectively [2,3]. For thyroid nodules that previously yielded nondiagnostic outcomes, the current guidelines suggest either repeating the FNA procedure or considering surgery if the nondiagnostic nodule presents an unclear cytological diagnosis [4].
Core needle biopsy (CNB) has emerged as a substitute for FNA that resolves several of the previously mentioned issues. CNB collects tissue samples with potential information on architectural histological structures and is useful for diagnosis of thyroid nodules [5]. Prior studies have illustrated that CNB significantly decreased the rates of nondiagnostic outcomes while simultaneously enhancing the accuracy of malignancy diagnoses in comparison with FNA cytology [6,7]. The American Association of Clinical Endocrinologists/American College of Endocrinology/Associazione Medici Endocrinologi (Italian Medical Endocrinologists Association) suggest the use of CNB in thyroid nodules with repeatedly nondiagnostic FNA, and the Korean Society of Thyroid Radiology extends the recommendations to nodules with indeterminate FNA or with troublesome cytological diagnosis [6,8,9].
Genetic modifications have been implicated in thyroid carcinoma, predominantly aberrant activation of the RAS-RAF-MEK-MAP signaling cascade [10]. Specific mutations in B-rapidly accelerated fibrosarcoma V600E (BRAF V600E) and rat sarcoma Q61R (RAS Q61R) are well-documented driver mutations in the pathogenesis of thyroid neoplasms [11]. A recent meta-analysis demonstrated that the integration of BRAF V600E assessment with FNA increased sensitivity by 6% while concurrently reducing the false-negative rate from 8% to 5.2% [12]. The incorporation of immunohistochemical (IHC) analysis of BRAF V600E and RAS Q61R expression status alongside CNB is posited to further amplify the diagnostic performance outcomes of the evaluation.
Despite the increase in CNB research, no studies from Indonesia have been documented on the diagnostic performance of thyroid CNB, including the utility of BRAF V600E and RAS Q61R IHC protein expression in identifying various thyroid nodules. CNB has been performed over the past 2 years at our institution. This study presents our initial experience with CNB, with a particular focus on its diagnostic performance and the utility of BRAF V600E and RAS Q61R IHC protein expression in the diagnosis of thyroid nodules.
MATERIALS AND METHODS
Selection of subjects
We gathered retrospective data from patients who underwent CNB for thyroid nodule at the Department of Anatomical Pathology, Dr. Cipto Mangunkusumo Hospital, Faculty of Medicine, Universitas Indonesia, between July 2022 and July 2024. In our institution, CNB is performed as a second-line diagnostic tool for thyroid nodule. Patients with thyroid nodules that warrant CNB are those with a clinically high suspicion for anaplastic carcinoma, medullary carcinoma, or thyroiditis; those with large thyroid nodules (>3 cm); those with nodules with sonographic characteristics of macrocalcification and hypervascularity; those that exhibit non-malignant FNA diagnosis while US findings show malignancy; and those with prior scanty, nondiagnostic, or inconclusive FNA aspirates.
CNB procedures were performed under US guidance and conducted by an endocrinologist, otorhinolaryngologist, or surgical oncologist with varying levels of experience in thyroid ultrasonography and interventional US. The CNB diagnosis is stratified into six categories based on the latest practice guidelines for thyroid CNB [13]: category I (nondiagnostic), category II (benign), category III (atypia of undetermined significance), category IV (follicular neoplasm), category V (suspicious for malignancy), and category VI (malignancy).
After excluding patients with inaccessible medical records, incomplete and/or disrupted hematoxylin and eosin–stained slides, or incomplete formalin-fixed paraffin-embedded (FFPE) tumor specimens, a total of 338 CNB samples was included in the study. Clinical data, including age, sex, location of thyroid nodule, CNB diagnostic category, and types of surgery performed, were obtained from medical records.
Of the 338 CNB samples, 104 were obtained from patients who underwent surgery with available postoperative diagnoses. The postoperative diagnosis was performed and re-reviewed by two board-certified endocrine pathologists (A.S.H. and M.F.H.) following the fifth edition of the World Health Organization classification of tumors [14]. The nodule size was determined as the largest diameter measured in the surgical specimen.
Immunohistochemistry examination of BRAF V600E and RAS Q61R
The expression of BRAF V600E and RAS Q61R proteins was assessed in 104 cases with postoperative diagnosis, irrespective of the CNB diagnostic category, using standard IHC procedures. We performed immunostaining on 4-µm-thick tissue sections from each FFPE tissue sample, which were the same as those used in the CNB diagnostic assessment [15,16]. The Optiview DAB IHC Detection Kit was used to perform immunostaining on a Starr Trek Universal HRP Detection (Biocare Medical, Concord, CA, USA) at the IHC Laboratory, Cipto Mangunkusumo Hospital, Jakarta. The manufacturers’ instructions (CC1 pretreatment for 32 minutes at 100°C, pH 8.5, antibody dilution at 1:600 for anti-BRAF V600E [mutated V600E] antibody [VE1] [ab228461, Abcam, Cambridge, UK] and 1:100 for anti-RAS [mutated Q61R] antibody [SP174] [ab227658, Abcam], incubation at 37°C for 16 minutes, examination using the Optiview DAB IHC Detection Kit) were followed.
Statistical analysis
Data were processed using Statistical Program for Social Science (SPSS) ver. 29 (IBM Corp., Armonk, NY, USA). Data on patient sex, CNB category, histological subtype, and other categorical data are provided as frequencies and percentages. Data on age and tumor size are presented as median values based on the distribution abnormality of the numerical data. We conducted statistical analysis using the Pearson Chi-Square test to compare CNB category with postoperative diagnosis. CNB categories I–III were regarded as non-neoplastic, while CNB categories IV–VI were regarded as neoplastic. The CNB groups were statistically compared based on postoperative diagnosis of neoplasm, which include lesions such as papillary thyroid carcinoma (PTC), invasive encapsulated follicular variant papillary thyroid carcinoma (IEFVPTC), differentiated high-grade thyroid carcinoma (DHGTC), poorly differentiated thyroid carcinoma (PDTC), oncocytic carcinoma, and follicular adenoma (FA). We also analyzed CNB categories in identifying malignant lesions; CNB categories I–IV were regarded as non-malignant and CNB categories V–VI were regarded as malignant. The diagnostic performances of CNB in identifying both neoplasm and malignant lesions at postoperative diagnosis were evaluated using sensitivity, specificity, accuracy, positive predictive value (PPV), negative predictive value (NPV), positive likelihood-ratio (LR), and negative LR. Further analyses were conducted to assess the diagnostic performance of CNB based on the IHC expression of BRAF V600E and RAS Q61R protein. Samples exhibiting positive BRAF V600E and RAS Q61R proteins were reclassified into neoplasm and malignant categories irrespective of their previous CNB classifications. Secondary analysis using binary logistic regression was used to assess the correlation between nodule size and postoperative diagnosis of both neoplasms and malignant lesions. A p-value less than .05 indicated statistical significance.
RESULTS
This study included 338 patients with thyroid nodules who underwent CNB. The demographic data of the patients are shown in Table 1. The mean age of the patients was 50.1 ± 16.1 years, and most were female (87.3%). CNB thyroid samples were predominantly collected from the right lobe of the thyroid gland (49.7%). The most common CNB category was II (52.7%), followed by IV, III, VI, I, and V, in this order (Fig. 1). The average core size was 0.65 ± 0.61 cm, and the average number of cores was 3.1 ± 2.1.
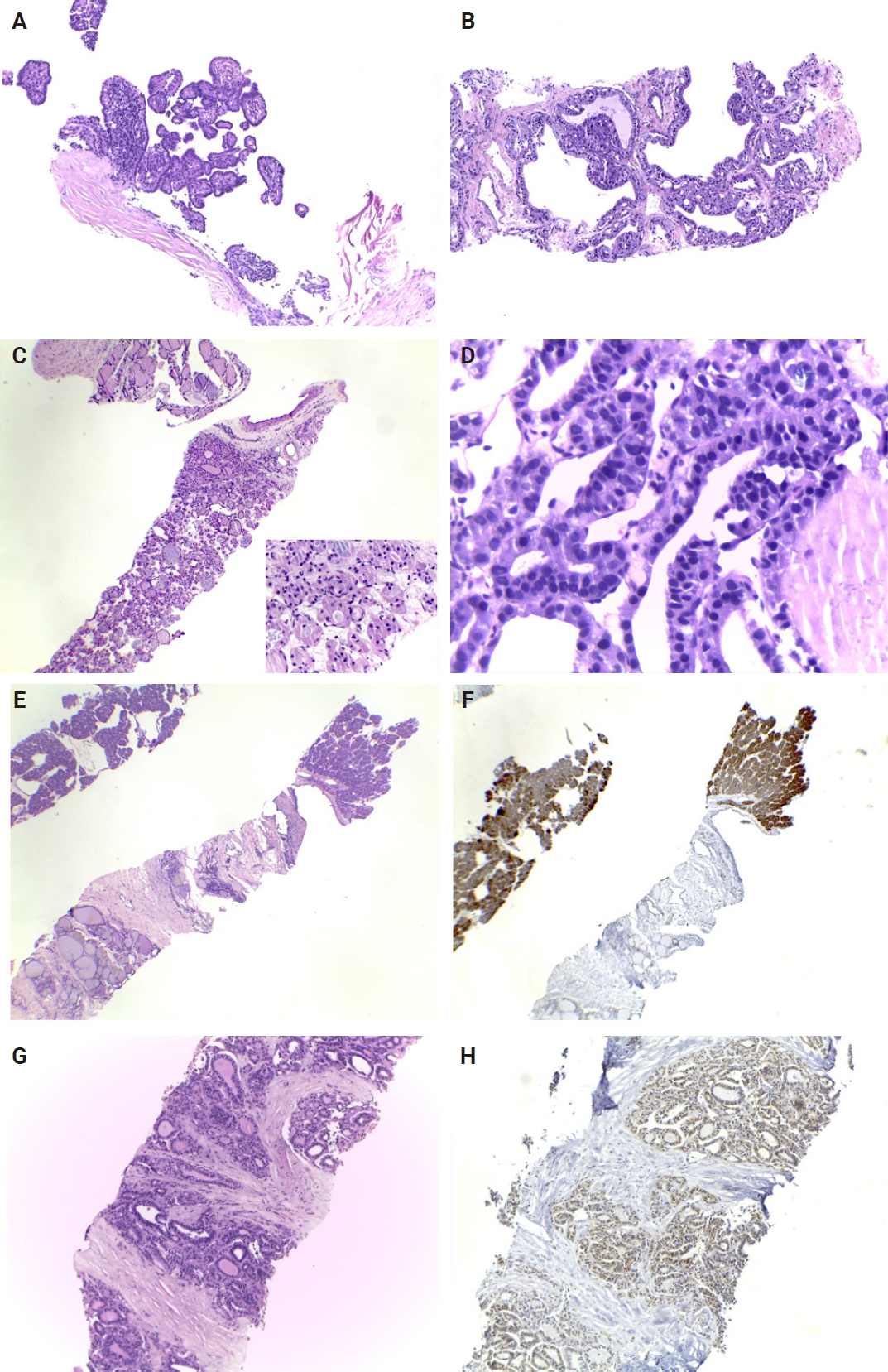
Representative cases of thyroid core needle biopsy. (A) Papillary thyroid carcinoma with a papillary architecture, containing a fibrovascular core and lined by densely packed elongated atypical cells, classified as category VI. (B) A case of Graves’ disease classified as category II, demonstrating hyperplastic follicles with hyperfunctioning cells and empty lumina. (C) A follicular neoplasm (category IV) exhibiting a microfollicular pattern and surrounded by a fibrous capsule. (D) Core needle biopsy showing tumor cells with enlarged atypical nuclei, condensed chromatin, and numerous bubble artifacts that should not be mistaken for true nuclear pseudoinclusions. (E, F) A case demonstrating a follicular pattern with fibrosis, histologically categorized as Bethesda category V. Further investigation revealed positivity for a RAS mutation (immunohistochemistry). (G, H) A case of follicular neoplasm with positive BRAFV 600E mutation (immunohistochemistry).
Postoperative diagnoses were achieved for 104 of 338 CNB samples (30.8%). Most patients underwent total thyroidectomy (81.7%). This study identified thyroid lesions including PTC, IEFVPTC, DHGTC, PDTC, oncocytic carcinoma, FA, and multinodular goiter (MG). PTC was identified in 59.6% of the postoperative samples, including infiltrative follicular, oncocytic, classic, tall cell, solid, and columnar subtypes. Three cases were identified as DHGTC in postoperative diagnosis, including one tall cell subtype of PTC, one follicular subtype of PTC, and one oncocytic carcinoma. The most common concurrent disease found in thyroid nodules samples was MG (45.2%). Thyroid nodule size was measured in postoperative histological examination, and the mean nodule size was 3.62 ± 2.9 cm.
In the 104 cases with postoperative diagnosis, CNB diagnosis of category IV was the most prevalent (39.4%), followed by category II (32.7%) and category VI (16.3%). Table 2 provides a detailed distribution of thyroid carcinoma diagnosis across CNB categories. Most PTC cases were classified as CNB category IV (46.8%), followed by category VI (25.8%) and category II (17.7%). The infiltrative follicular subtype was the most common PTC subtype, with 48% of cases classified as CNB category IV. The oncocytic subtype of PTC cases was predominantly categorized as category IV (69.2%). We found eight cases of tall cell subtype of PTC; most were classified as category VI (87.5%). The only oncocytic carcinoma case was category IV. All three cases of DHGTC were CNB category IV. Most PDTC and IEFVPTC cases were category IV (50% and 40%, respectively). FA cases were classified as category IV (50%), II (25%), and III (25%), while all MG cases were category II.
Comparison of thyroid CNB categories with postoperative neoplastic diagnosis
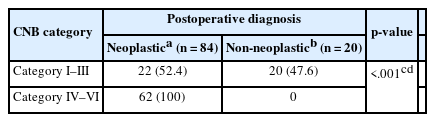
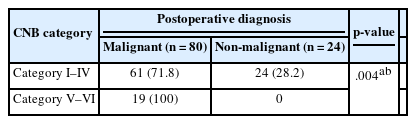
Table 3 shows the significant association between CNB category and postoperative neoplastic diagnoses (p < .001). CNB categories IV–VI (follicular neoplasm, suspicious for malignancy, and malignant) were significantly associated with neoplastic lesions in postoperative diagnosis.
Comparison of thyroid CNB categories with postoperative malignancy diagnosis
Table 4 shows the relationship between the CNB category and postoperative malignancy diagnosis. CNB categories V–VI (suspicious for malignancy and malignant category) were more frequently associated with malignancy in postoperative diagnosis. This result highlights the limitations of CNB to rule out malignancy in CNB category I–IV and indicate the need for caution with such lesions, particularly in indeterminate or follicular neoplasm cases.
Diagnostic performance of CNB
We further compared the diagnostic performance of CNB in detecting both neoplastic and malignant lesions. Table 5 showed that CNB exhibited a higher sensitivity and overall accuracy in detecting neoplasms than malignant lesions (74% vs. 23.8% and 79% vs. 41.4%, respectively). When combined with BRAF V600E IHC, CNB showed superior sensitivity and overall accuracy in detecting neoplasms compared with malignant lesions (74% vs. 28.8% and 79% vs 44.4%, respectively). When combined with RAS Q61R IHC, CNB showed better sensitivity and overall accuracy in detecting neoplasms than malignant lesions (77% vs. 23.8% and 82% vs 41.4%, respectively). Other parameters such as specificity, PPV, and NPV showed no significant difference for detection of neoplasms and malignant lesions for CNB alone, CNB and BRAF V600E IHC, CNB and RAS Q61R, and CNB and BRAF V600E/RAS Q61R.
Association between nodule size and postoperative diagnosis
We further examined the association between thyroid nodule size and postoperative neoplastic diagnosis (Table 6). The analysis showed that nodules >3 cm in diameter were associated with neoplastic lesions in postoperative diagnosis (p =.005; odds ratio [OR], 8.19).
Table 7 displays the relationship between thyroid nodule size and postoperative malignant diagnosis. We found that thyroid nodule >3 cm in diameter was significantly associated with malignant lesion (p = .004; OR, 5.83).
DISCUSSION
In the present study involving 338 thyroid nodule patients, the most common CNB diagnosis was category II (52.7%), followed by category IV (15.1%). Category II indicates a benign lesion, which includes non-thyroid lesion or benign thyroid lesions, including Hashimoto thyroiditis or benign follicular nodules. Category IV refers to follicular thyroid neoplasms, including the conventional type, which may or may not exhibit nuclear features, and Hurthle cell neoplasm [5]. The management approach of thyroid nodule is multidisciplinary; however, the cytology findings of category II typically necessitate a watchful follow-up, often without the need for surgical intervention. This may explain why, among 338 CNB thyroid nodules, only 30.8% received postoperative diagnoses.
Most patients with postoperative diagnosis in this study underwent total thyroidectomy and were classified as CNB category IV. While other clinical considerations may suggest the need for surgical intervention, CNB category IV is not indicated for total thyroidectomy [5]. However, most category IV cases were identified as PTC in postoperative diagnosis, warranting further discussion. A previous study found that a significant proportion of category IV cases in CNB samples exhibited varying risk of malignancy (ROM), with those presenting nuclear atypia showing a higher ROM (40%–62%) [17]. Jung et al. [5] noted that, within category IV, there is a potential for noninvasive follicular thyroid neoplasm with papillary-like feature or an invasive follicular variant of PTC (NIFTP), particularly in those with some nuclear features typical of PTC. A study by Haq et al. [18] also highlighted the need to carefully evaluate the presence of nuclear features that suggest a diagnosis of PTC in category IV. From the substantial findings from previous studies and our cohort, the reclassification of category IV warrants consideration to improve diagnostic accuracy and clinical management.
This study identified several histological subtypes of PTC including classic, tall cell, solid, oncocytic, columnar, and infiltrative-follicular subtypes. Notably, the relative incidence of PTC subtypes in this study differs from a prior study conducted on all resection specimens diagnosed at our institution [11]. In contrast to findings in the study by Harahap et al. [11], the infiltrative follicular subtype was more prevalent than classic PTC in CNB samples with available postoperative diagnosis. This may result from a greater proportion of the classic subtype of PTC being detected early since the papillary architecture of the classic subtype is readily identifiable in FNA. While subtypes such as classic, infiltrative follicular, oncocytic, and solid are predominantly reported as category IV, the tall cell subtype of PTC is frequently presented as category VI (malignancy). The most common pitfall of diagnosing PTC in CNB material is false-negative identification of nuclear features, which is attributable to the smaller and darker chromatin in CNB aspirates compared with surgical specimens [5]. Nevertheless, previous research indicated that the majority of the tall cell subtype exhibits tall columnar cells in CNB specimens, with 41% of samples demonstrating 30% of tall columnar cells, indicative of a tall cell diagnosis [19].
In addition to PTC, we identified other malignant thyroid lesions, including IEFVPTC, DHGTC, PDTC, and oncocytic carcinoma, as well as benign thyroid lesions such as FA and non-neoplastic thyroid lesions like MG in the postoperative diagnosis. IEFVPTC is an encapsulated follicular subtype of PTC with invasion and was commonly reported as category IV and category I in the present study. We identified one IEFVPTC case that was reported as category VI. The obscure manifestation of nuclear atypia, along with the lack of tumor invasion, complicates the identification of IEFVPTC in biopsy materials. Despite the predominance of cases in category IV in this study, no NIFTP cases were identified in postoperative diagnoses. The phenomenon can be explained by the rare occurrence of NIFTP in our institutional setting, along with the limited number of thyroid nodule cases included in this study.
DHGTC and PDTC are both rare and underrecognized neoplasms, accounting for less than 3% of all thyroid malignancies [20,21]. Three cases were diagnosed as DHGTC in the present study: the PTC tall cell subtype, the PTC follicular subtype, and oncocytic carcinoma. All DHGTC cases in this study were reported as CNB category IV. In one case of the DHGTC tall cell subtype, the tall cell component was estimated to constitute roughly 30% of the area, while most of the structure was largely follicular. In line with our present finding, oncocytic carcinoma is a rare type of thyroid neoplasm originating from oncocytic cells of the thyroid gland and is commonly reported as category IV in biopsy [22]. Distinguishing oncocytic carcinoma from adenoma, however, requires evidence of capsular and vascular invasion, which is challenging to assess in biopsy aspirates.
The second most common CNB diagnosis in patients with postoperative diagnosis was category II. Notably, MG constituted 58.8% (20 of 34) of diagnoses in category II (benign lesion). A prior study indicated that category II exhibits the lowest ROM among CNB categories, with values between 2%–6%, as determined by final diagnosis through clinical and/or surgical follow-up [13]. This finding emphasizes the necessity for a careful reevaluation of surgical treatment options for category II, except in cases requiring urgent airway management.
In comparison with US-FNA, CNB has lower rates of inconclusive results [23-26]. Approximately, 20%–30% of FNA are nondiagnostic and require repeated FNA or are treated with unnecessary lobectomy [27-29]. With the use of the relatively bigger gauge needle, CNB is considered more effective at obtaining larger tissue samples than the FNA procedure and allows cytological and architectural evaluation of tumor samples. The present study supports previous findings in which we found a low proportion of inconclusive CNB results. Category I (nondiagnostic) and category III (atypia of undetermined significance) represent only 7.1% and 12.1% of all CNB specimens in the study, respectively. However, compared with category II, categories I and III typically display higher ROM, ranging from 18%–50% and 32%–45%, respectively [13]. A significant proportion of category III cases is differentially diagnosed as category IV or V because of the obscure presence of nuclear or oncocytic atypia, a small amount of tumor cells, and the conflicting presence of tumor capsule [13,30]. Similarly, in the present study, all CNB category I cases were diagnosed postoperatively as either PTC or IEFVPTC, whereas CNB category III may be diagnosed postoperatively as FA, PTC, or aggressive PDTC. Therefore, meticulous attention with ongoing monitoring and repeat biopsy procedure is recommended.
In the present study, we found a significant association of CNB category with neoplastic and malignant lesions. CNB achieved a sensitivity of 74% and specificity of 100% in detecting neoplastic lesions. In contrast, CNB yielded a sensitivity of 23.8% and specificity of 100% for detecting malignant lesions. Previous studies reported that the CNB sensitivity for detecting malignancy in thyroid nodules was greater than 90%, with a specificity ranging from 90%–100% [2,3,31,32]. A recent meta-analysis revealed a wider range of CNB sensitivity, ranging from 44.7–85%, with a specificity of 100% [29]. CNB diagnostic performance varies in prior publications, with consistently high specificity but lower sensitivity. While a CNB diagnosis of category V and VI represents true thyroid malignancy in surgical diagnosis, categories I–IV could not exclude the possibility of thyroid malignancy. This is particularly significant as the exclusion of critical features such as vascular and capsular invasion may limit the accuracy of malignancy diagnosis in these categories. The same rationale applies to CNB performance in identifying neoplastic lesions.
This study demonstrated a lower sensitivity and higher specificity for neoplastic lesions, indicating that CNB categories IV–V are specific for neoplastic lesions, and the presence of neoplastic lesions remains possible in CNB categories I–III. This phenomenon may be explained by the non-representative and low-cellularity samples obtained from CNB procedures compared with surgical specimens.
CNB allows IHC examination that aids in diagnosis and predicting tumor behavior [29]. Recent studies reported on the use of molecular testing to identify thyroid nodules in CNB specimens, particularly when initial CNB results are indeterminate [33-35]. In the study by Jung [13], in CNB samples where histologic morphologies indicate a differential diagnosis of categories III and IV, a positive result of RAS Q61R IHC simplifies the decision favoring categorization into category IV. BRAF V600E IHC is useful when CNB samples display nuclear atypia yet lack sufficient histologic features for definitive malignancy features. These cases may be assigned to category III or category V based on the extent of nuclear atypia and the quantity of atypical cells involved. A positive result of BRAF V600E IHC in indeterminate CNB results typically points toward a definitive diagnosis of PTC [13]. IHC is economical, feasible, and sensitive for detecting BRAF V600E and RAS Q61R mutations in thyroid nodules [36]. A previous study revealed a sensitivity of 100% and specificity of 42.86% of IHC for detecting BRAF V600E mutation [37]. Thus, IHC would be beneficial as a preliminary screening method to detect BRAF V600E and RAS Q61R mutations [37]. Moreover, Crescenzi et al. [38] found that IHC performed on CNB samples of thyroid nodules perfectly matched the genetic analysis of BRAF V600E status.
To the best of our knowledge, this is the first study that incorporated IHC of BRAF V600E and RAS Q61R in addition to CNB. In evaluating the performances for detecting neoplastic lesion by incorporating BRAF V600E IHC, RAS Q61R IHC, or both with CNB, the sensitivity was 74%, 77%, and 77%, respectively. The specificity was 100% for all analyses, while the overall accuracy was 82%. For detection of malignant lesions, incorporating BRAF V600E IHC increased the sensitivity to 28.8% and overall accuracy to 44.2%, while RAS Q61R IHC did not enhance the diagnostic performance of CNB. In our cohort, categories II, IV, and VI remained unchanged with respect to BRAF V600E and RAS Q61R IHC results, while three samples previously classified as category III based on CNB examination alone were reclassified into category IV and one sample initially classified as class V was reclassified as class VI based on BRAF V600E IHC results. The sensitivity increase was not significant compared with that of CNB examination alone, indicating that IHC staining is not clinically meaningful in differentiating neoplasms in thyroid lesions.
In our cohort, the thyroid nodule size was larger than 3 cm, which showed a significant association with neoplastic and malignant postoperative diagnosis and higher odds ratio of being neoplastic and malignant at postoperative diagnosis. Hong et al. [20] stated that malignancy risks increased as the nodule size increased in low- and intermediate-suspicion nodules determined by US results. The malignancy rate of large nodules (≥3 cm) was higher than that of small nodules (<3 cm) in intermediate-suspicion nodules (40.3% vs. 22.6%; p = .001) and low-suspicion nodules (11.3% vs. 7.0%; p = .035) [20]. In agreement, Hahn et al. [21] reported that thyroid nodules larger than 2 cm are an important factor in the superiority of CNB to FNA in the detection of low-to-intermediate lesions from US. Large nodules are often heterogeneous and contain areas of both benign and malignant tissues, more complex architecture, and higher proportions of cystic areas; samples obtained by FNA were inadequate for interpretation, leading to higher false-negative results [39].
While the performance of CNB in detecting thyroid neoplastic lesions is satisfactory, its detection of malignant thyroid lesions has not yet reached optimal levels at our institution, particularly compared with prior studies. We attribute this primarily to the limited number of cases involved, limited duration of CNB implementation, and operator skillsets and expertise. Ahn et al. [40] stated that the diagnostic results of CNB may differ by pathologist, operator, and institution.
This study has several limitations. First, it was a retrospective study performed in a tertiary hospital in Indonesia. Thus, there might be concerns of patient selection bias, and the results may not reflect the entire general population. This study did not evaluate operator variability and ultrasonographic features in the performance of CNB. The number of samples with postoperative diagnosis in our study was not large compared with previous studies that included hundreds to thousands of participants. This is because CNB has been applied in our institution only for the previous 2 years, and there have been few cases. However, these limitations can be overcome with prospective, randomized-controlled trials and multicenter studies.
The present study demonstrated a lower rate of inconclusive results and a higher category IV CNB diagnostic rate in the diagnosis of thyroid nodules for CNB compared to FNA. The diagnostic performance of CNB in detecting malignancy was relatively poor, while its performance for detection of neoplastic lesions was stronger.
Notes
Ethics Statement
This study was approved by the Faculty of Medicine at Universitas Indonesia's Institutional Review Board and performed following the 2013 revision of the Declaration of Helsinki (IRB No: KET-1316/UN2.F1/ETIK/PPM.00.02/2023). Informed consent was waived by the board (No-602/UN2.F1/ETIK/PPM.00.02/2024).
Availability of Data and Material
All data analyzed during this study are included in this published article.
Code Availability
Not applicable.
Author Contributions
Conceptualization: ASH, MFH, TJET, RAW, EDJ. Data curation: ASH, MFH, TJET, EDJ, RI, CIAM. Formal analysis: ASH, TJET, RAW, EDJ, RI, CIAM. Funding acquisition: ASH, MFH, TJET, RAW, EDJ. Investigation: ASH, MFH, TJET, EDJ. Methodology: ASH, TJET, RAW, EDJ. Project administration: RI, CIAM. Resources: ASH, MFH, TJET, RAW, EDJ. Supervision: MFH, EDJ. Validation: ASH, MFH, TJET, RAW, EDJ. Visualization: ASH. Writing – original draft: ASH, RI. Writing – review & editing: ASH, MFH, TJET, RAW, EDJ, RI, CIAM. Approval of final manuscript: all authors.
Conflicts of Interest
The authors declare that they have no potential conflicts of interest.
Funding Statement
This research was funded by Dr. Cipto Mangunkusumo Hospital Operational and Innovation Research Grant 2024.