Acquired aberrant partial CD3 expression in recurrent Epstein-Barr virus–negative solitary plasmacytoma of tonsil
Article information
Abstract
The aberrant expression of specific T-cell maker CD3 in B-cell neoplasms can be a potential diagnostic pitfall leading to a misclassification of cell lineage. Here, we report a case of recurrent solitary plasmacytoma with new aberrant expression of CD3. The neoplastic plasma cells of the recurrent tumor were kappa restricted, positive for CD138, MUM1, negative for CD20, cyclin D1, and Epstein-Barr virus. CD79a was positive in majority of the tumor cells, except for a small focus which was strongly positive for CD3, but negative for other T-cell markers (CD2, CD5, CD7, CD4, and CD8) and CD56. The neoplastic plasma cells of the original tumor were negative for CD3. To the best of our knowledge, only one case of recurrent plasmacytoma with aberrant expression of CD3 has been published, which revealed disease progression in the recurrence. However, we did not observe morphologic evidence of disease progression in our case.
INTRODUCTION
CD3 is a specific marker of T-cell lineage lymphoma [1]. However, aberrant expression of CD3 has been reported in rare cases of B-cell neoplasms including diffuse large B-cell lymphoma (DLBCLs) [2,3], classic Hodgkin’s lymphoma [4], anaplastic lymphoma kinase–positive large B-cell lymphoma [5], plasmablastic lymphomas (PBLs) [6,7], plasma cell neoplasms [8,9], primary effusion lymphoma [10], and primary mediastinal (thymic) large B-cell lymphoma [11]. It has been reported that Epstein-Barr virus (EBV) may promote lineage ambiguity/infidelity and possibly play a role in the aberrant expression of CD3 in B-cell neoplasms [11]. However, the hypothesis of the mechanism of EBV is controversial [3,6,12]. Downregulation of B-cell transcription factors, especially PAX5 is another proposed mechanism [13].
The aberrant expression of CD3 in PBL, plasmablastic transformation of myeloma, and plasmacytoma could make the diagnosis more difficult because the cells are usually negative for common B-cell markers (CD20, CD79a, and PAX5) [6,14]. In cases with only subtle plasmacytic or plasmablastic morphology, and in cases with poorly preserved tumor cells, the aberrant expression of T-cell antigens can potentially lead to a misdiagnosis of T-cell lymphoma [6,11]. In addition, studies suggest that the aberrant expressions of CD3 observed in relapsed plasmacytoma [15], recurrent multiple myeloma [8], and plasmablastic transformation of plasma cell myeloma (PCM) [16] are associated with the poor prognosis represented as high-grade transformation of the tumor cells and aggressive clinical course. Therefore, it is important and practical for pathologists to be aware of this rare phenotype of aberrant expression of T-cell antigens in B-cell neoplasms to avoid the potential diagnostic pitfall and to elucidate the correct diagnosis.
To the best of our knowledge, only one case of recurrent plasmacytoma with aberrant expression of CD3 has been published [15]. Herein, we report a second rare case of recurrent plasmacytoma located in the right tonsil with the aberrant expression of CD3 in a focus of the malignant plasma cells. The clinical course has been described and the morphology, the immunohistochemistry (IHC) phenotypes have been compared between the initial tumor and the recurrence.
CASE REPORT
Our patient is a 66-year-old male with recurrent plasmacytoma. He initially presented at a local otolaryngology clinic due to loss of smell and taste for about 2 years. On flexible nasopharyngoscopy, a 1.5 cm, exophytic, red mass at right posterior tonsillar pillar was found. A direct laryngoscopy with biopsy was done in the fall of 2023 and pathology revealed a kappa-restricted plasmacytoma. Computed tomography (CT) sinus without contrast showed normal paranasal sinuses. Bone marrow biopsy showed no evidence of a monoclonal plasma cell population. No M-spike was detected by serum protein electrophoresis; both kappa and kappa/lambda ratio were mildly elevated. Immunoglobulins showed normal IgG and mildly elevated IgA. Positron emission tomography/CT was done at the end of 2023 and showed no evidence of asymmetric hypermetabolic mass in the oropharynx, only some symmetric hypermetabolic prominence of bilateral palatine and lingual tonsils were detected, which were favored to be infectious/inflammatory in etiology. Given solitary presentation, the patient received image-guided intensity-modulated radiation therapy with 50.4 grays for 5 weeks, which was completed at the beginning of 2024. About 2 months after the completion of the radiation therapy, the radiation oncologist noted a recurrent mass in the right tonsil fossa. In the spring of 2024, the patient presented to the otolaryngology department of the University of California Irvine Medical Center (UCIMC) for a follow-up check. Physical exam of the oral cavity/oropharynx revealed a 1 cm red exophytic rubbery mass extending to the right tonsillar bed. Some post-operation changes such as fibrinous exudate along the right pharyngeal bed were appreciated as well. The patient underwent transoral robotic surgery of right pharyngectomy and pharyngoplasty.
The histologic findings of the surgically resected tumor revealed multifocal plasmacytic proliferation in the submucosa area of pharyngeal squamous mucosa. The neoplastic plasma cells appeared to be well to moderately differentiated with no plasmablastic features and were diffusely positive for MUM1 and CD138, while negative for CD20 and cyclin D1 (Fig. 1A–H). A low proliferative index (<5%) was detected by Ki-67 (Fig. 1I). The neoplastic plasma cells were kappa restricted and were negative for Epstein-Barr encoding region (EBER) in situ hybridization (ISH) (Fig. 1J–L). CD79a was strongly positive in the neoplastic plasma cells except for a small focus of cells with significantly reduced expression (Fig. 2A–C). In addition, only the neoplastic plasma cells in this small area were diffusely and strongly positive for CD3 and epithelial membrane antigen (EMA), with weak expression of IgG (Fig. 2D–H). These tumor cells with aberrant expression of CD3 were also kappa restricted (Fig. 2I, J). The staining intensity of the aberrant expression of CD3 in the neoplastic plasma cells was dominantly cytoplasmic (Fig. 2E). In contrast, the dominant membranous staining intensity of CD3 was observed in the scattered normal T cells (Fig. 2F). Although the morphology of these CD3-positive neoplastic cells was plasmacytic, this unusual phenotype warranted us to repeat CD3 IHC and stain more T-cell markers to rule out the possibility of T-cell lymphoma. The repeated IHC of CD3 was strongly positive in the small focus of plasma cells but all the other T-cell markers (CD2, CD4, CD5, CD7, and CD8) were negative (Fig. 2K–O). CD56 was negative in the neoplastic plasma cells and only highlighted rare natural killer cells (Fig. 2P). The specimen was also sent for fluorescence in situ hybridization analysis, which showed immunoglobulin heavy chain (IGH) gene rearrangement that did not translocate to one of the common plasma cells dyscrasia translocation partners (fibroblast growth factor receptor 3, cyclin D1, MAF, or MAFB).
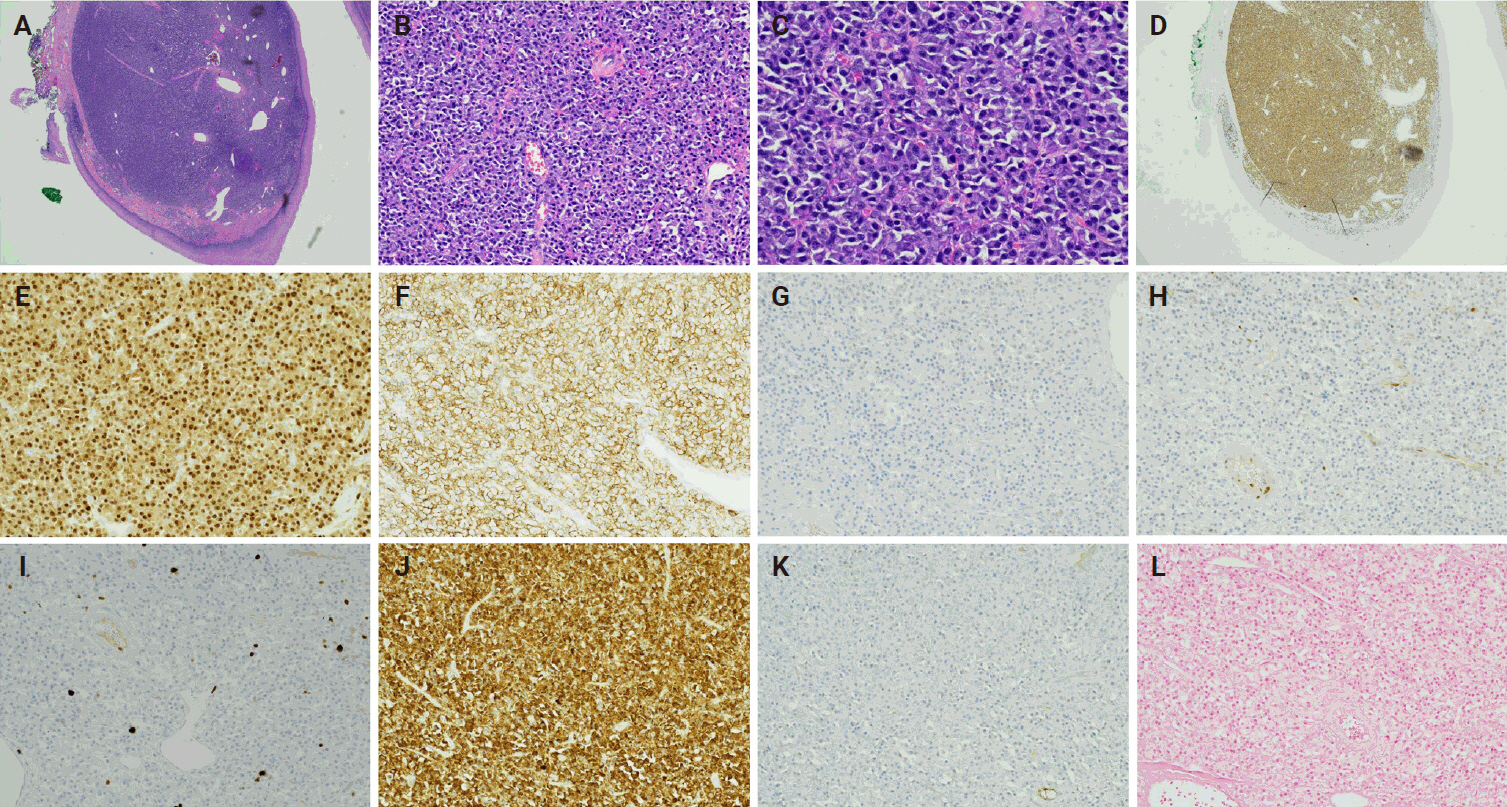
(A-C) Hematoxylin and eosin–stained slides from the recurrent plasmacytoma show well to moderately differentiated plasma cells. The neoplastic plasma cells are positive for MUM1 (D, E) and CD138 (F), negative for CD20 (G) and cyclin D1 (H). Proliferative index is about 5% by Ki-67 (I). The immunohistochemical staining is diffusely positive for kappa (J) while negative for lambda (K) which indicates the neoplastic plasma cells are kappa restrictive, and negative for Epstein-Barr encoding region in situ hybridization (L).
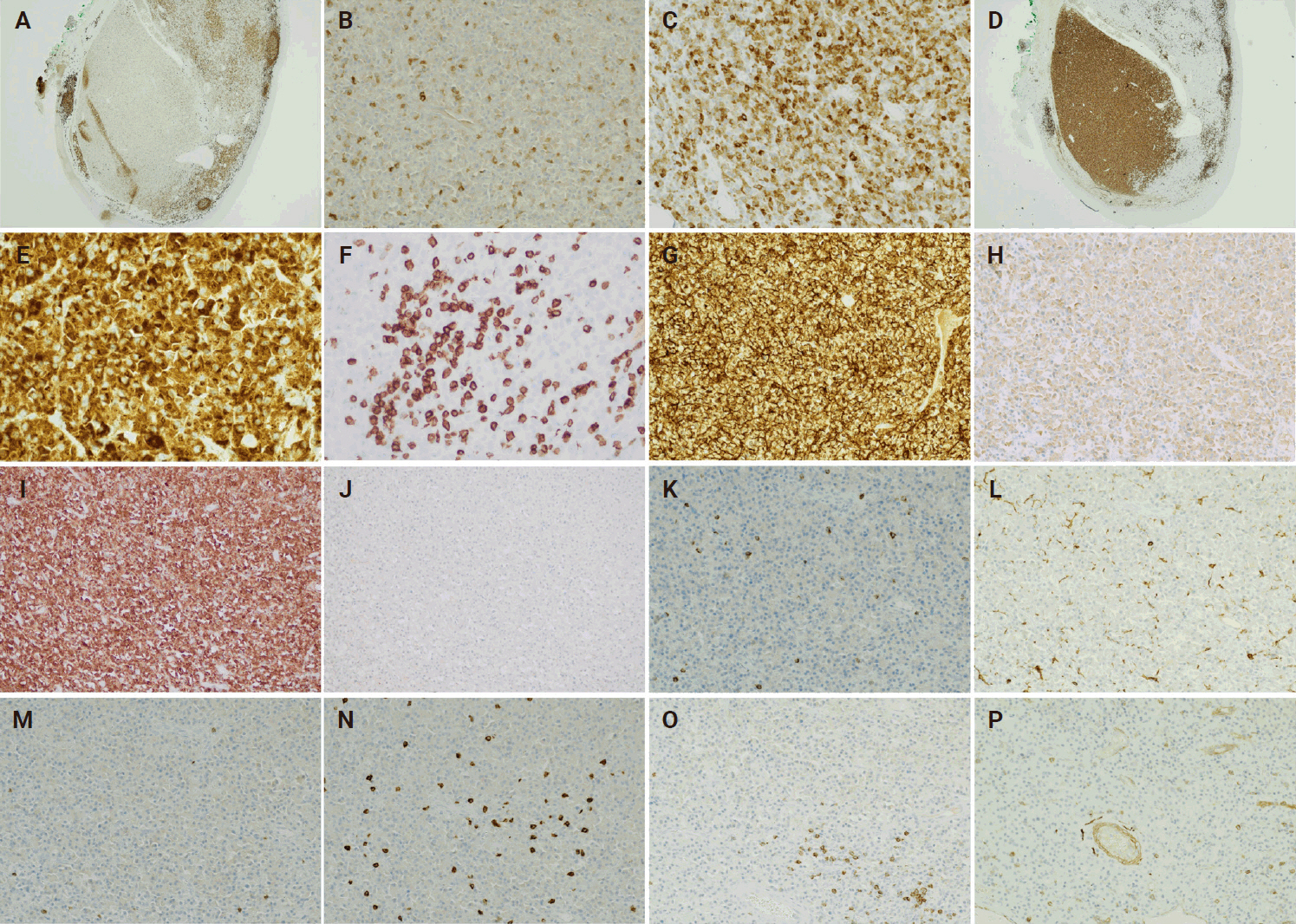
One focus of the neoplastic plasma cells in the recurrent plasmacytoma is with markedly reduced expression of CD79a (A, B), strongly positive for CD3 (D, E). CD79a is strongly positive in the CD3 negative plasma cells (C). CD3-positive plasma cells are positive for epithelial membrane antigen (G) with weak expression of IgG (H) and are the immunohistochemical staining is diffusely positive for kappa (I) while negative for lambda (J) which indicates the neoplastic plasma cells are kappa restrictive. Dominant cytoplasmic staining intensity of CD3 is in the neoplastic plasma cells (E) and dominant membranous staining intensity of CD3 is in the scattered normal T cells (F). There are no expressions of CD2 (K), CD4 (L), CD5 (M), CD7 (N), CD8 (O), and CD56 (P) in the neoplastic plasma cells of the recurrent plasmacytoma.
The initial biopsy of the tumor obtained from the patient in the fall of 2023 was subsequently reviewed at UCIMC. The neoplastic plasma cells appeared to be well to moderately differentiated, diffusely positive for CD138 and kappa restricted (Fig. 3A–F). To compare with the recurrence, CD2, CD3, CD20, CD79a, Ki-67, and EMA were performed on the initial biopsy. The results showed that CD79a was positive in the neoplastic plasma cells of the original tumor, but CD2, CD3, and CD20 were all negative (Fig. 3G–J). There was weak expression of EMA in the tumor cells (Fig. 3K). The proliferative index by Ki-67 was low (5%) (Fig. 3L). The diagnosis of extramedullary plasmacytoma with no aberrant expression of CD3 was confirmed.
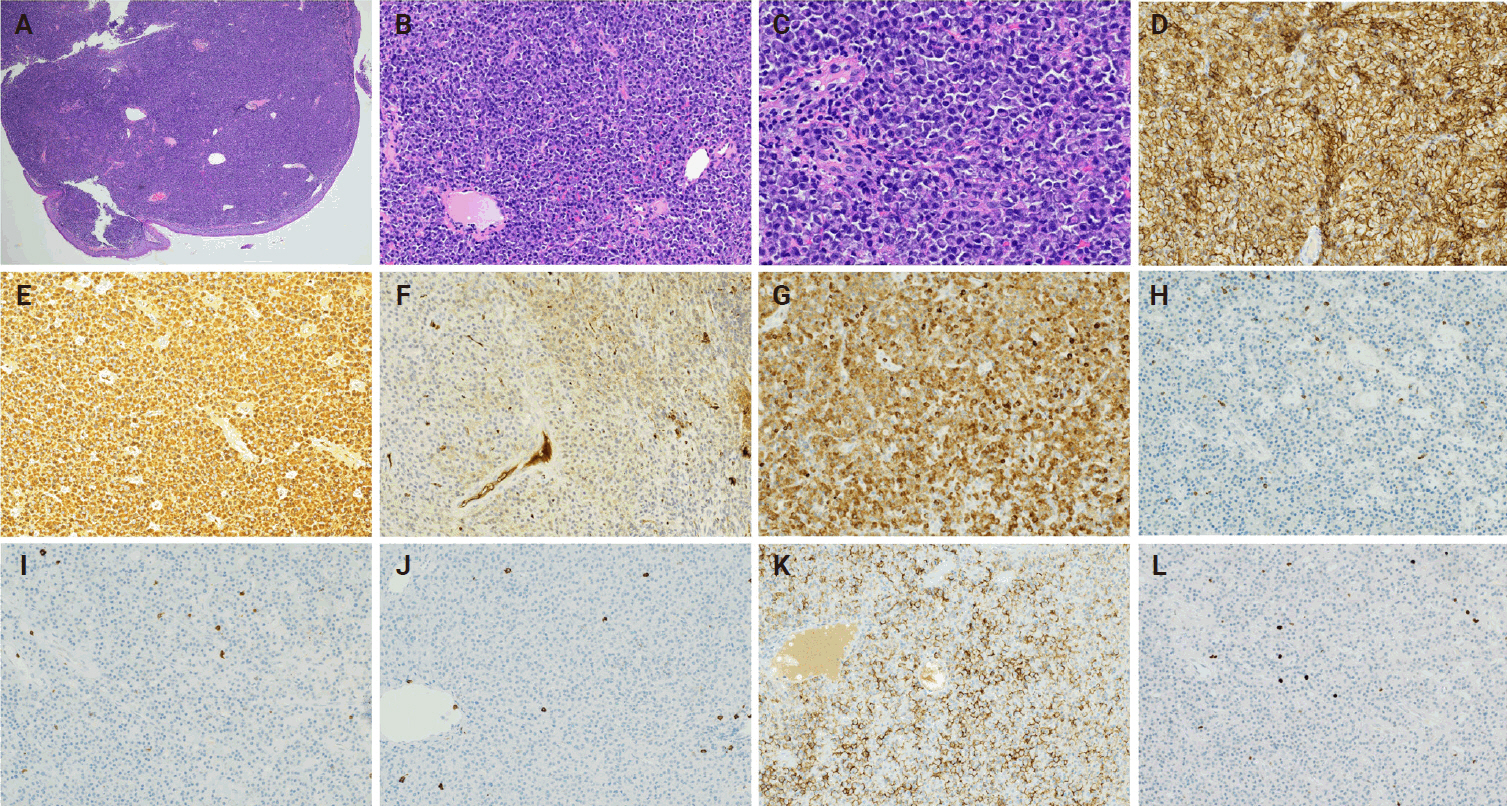
(A-C) Hematoxylin and eosin–stained slides from the original plasmacytoma show well to moderately differentiated plasma cells. The neoplastic plasma cells are positive for CD138 (D) and the immunohistochemical staining is diffusely positive for kappa (E) while negative for lambda (F) which indicates that the neoplastic plasma cells are kappa restrictive. The tumor cells are positive for CD79a (G), negative for CD2 (H), CD3 (I), CD20 (J), with weak expression of epithelial membrane antigen (K). Proliferative index is about 5% by Ki-67 (L).
DISCUSSION
We report a rare case of recurrent plasmacytoma of the right tonsil with aberrant expression of T-cell antigen CD3. To the best of our knowledge there is only one reported case of recurrent plasmacytoma of the thoracic vertebral body with aberrant expression of CD3 [15]. In the first case report, the histologic findings of the initial solitary plasmacytoma showed differentiated plasma cells with no aberrant expression of CD3. However, multiple recurrent tumors were found on magnetic resonance imaging including bone, muscle, lymph node, soft tissue of right forearm, both thighs, and lower left abdomen. The relapsed tumors were composed of medium-sized and mononuclear blastic cells mixed with smaller cells with plasmacytic differentiation consistent with plasmablastic myeloma. All the neoplastic cells of the recurrent tumor were diffusely positive for CD3. These cells were also positive for CD56, CD10 with high proliferative index approximately 80% by Ki-67. In addition, both the tumor cells of the original solitary plasmacytoma and the recurrent multiple tumors were positive for EBER ISH. All the features of relapsed plasmacytoma in the first case report represented a higher-grade morphology with a new immunophenotype of aberrant CD3 expression. Thus, the authors hypothesized the expression of CD3 could be possibly associated with disease progression and poor prognosis.
Our case also presented as recurrent plasmacytoma with aberrant expression of CD3; however, the clinical presentation and histologic features were different from the first case report. In our case, both the original tumor and the recurrence were solitary lesions. The morphologies of the neoplastic plasma cells of both tumors were well to moderately differentiated without plasmablastic features in the recurrent one. The CD3 was strongly positive in a small focus of the neoplastic plasma cells of the recurrent tumor with dominantly strong staining intensity in the cytoplasm, but negative in the rest of the tumor. The dominantly membranous positivity of CD3 was observed in the scattered normal T cells. Other T-cell markers such as CD2, CD4, CD5, CD7, and CD8 were absent in the tumor cells of the small focus, therefore the CD3 expression was the sole aberrant T-cell marker in our case, which is consistent with the published data [2,6,12]. There was no expression of CD3 in the neoplastic plasma cells identified by IHC in the initial biopsy of the original tumor. Thus, the aberrant expression of CD3 could be a new feature of the recurrence. However, due to the focal expression of CD3 in the recurrent tumor, the possibility of sampling variation leading to lack of detection of CD3 expression in the initial biopsy could not be completely ruled out. The recurrent tumor was negative for EBER ISH, but the status of EBV infection of the original tumor was not checked. Based on the morphology and clinical behavior of the recurrent tumor, we did not observe clinical evidence of disease progression.
Oliveira et al. [12] studied 21 cases of B-cell neoplasms with aberrant expression of CD3 including 12 DLBCLs, two PBLs, two plasmacytomas, one myeloma with plasmablastic features, one anaplastic myeloma, two Burkitt lymphomas, and one nodal follicular lymphoma. Their results demonstrated that plasmacytic differentiation was found in more than half (~62%) of the CD3-positive B-cell neoplasms, typically involving extranodal sites. Expression of CD3 in the cytoplasm was the sole aberrant T-cell marker in most of the cases with plasmacytic differentiation. In contrast, DLBCLs with anaplastic feature in this series showed aberrant membranous expressions of multiple T-cell antigens (CD3, CD2, CD4, CD5, and CD8) and exclusively involved lymph nodes. Another earlier study also demonstrated the membranous staining pattern of CD3 with co-expression one or more T-cell antigens in DLBCL [2]. Pan et al. [6] studied 17 cases of CD3-positive PBL and PCM with plasmablastic morphology. Their results indicated that these B-cell neoplasms with plasmablastic morphology and a high proliferative rate occurred in the extranodal sites predominantly in male patients and had moderate to strong cytoplasmic CD3 expression. However, other T-cell–associated markers were nearly absent. Therefore, they demonstrated that CD3 expression in the plasmablastic neoplasms was mostly cytoplasmic and a sole aberrant T-cell marker. They also showed most plasmablastic neoplasms in their study had kappa or lambda light chain restriction and/or clonal IGH rearrangements with no clonal T-cell receptor gamma (TRG) rearrangements, which essentially confirmed the B-cell lineage and excluded the possibility of a T-cell neoplasm. Among all the published cases of plasma cell neoplasms with aberrant expression of CD3, more cases show plasmablastic features, which is not observed in our case [6,15,16]. Very few cases of PCM without plasmablastic morphology with the aberrant expression of CD3 have been reported [9,12]. Dual expressions of CD3 and CD4 were also reported in the plasma cell neoplasms [16-18].
The mechanism of aberrant expression of CD3 in the B-cell neoplasms is still unclear. One hypothesis is effect of EBV infection. It has been reported that EBV might promote T-cell antigen expression in B-lineage neoplasms [19]. Although EBV infection has been reported in most B-cell neoplasms with aberrant expression of CD3 [11], only three out of 20 cases (2 PBLs, 1 DLBCL with plasmacytic differentiation) in the study of Oliveira et al. [12] were positive for EBV, and 10 out 25 plasmablastic neoplasm had EBV infection in the study of Pan et al. [6]. Wu et al. [3] also observed three cases of DLBCL with aberrant expression of CD3 that were negative for EBV infection. In addition, EBER ISH in our case was negative, which indicated it was likely EBV-independent. Therefore, EBV infection may only partially account for the aberrant expression of CD3 in B-cell neoplasms. Another hypothesis is downregulation of B-cell transcriptional factors, especially PAX5 which is an important regulator of B-cell differentiation [6]. It has been reported that loss of PAX5 in plasmacytic neoplasms may promote the aberrant expression of T-cell antigens [13].
Some studies also correlate the aberrant expression of T-cell markers with disease progression and poor prognosis. Siper et al identified six cases out of 215 cases of PCM with aberrant expression of T-cell antigens (4 with CD4, 1 with CD3, and 1 with CD2) [8]. Five out of six cases were relapsed tumors and showed shorter survival after the demonstration of T-cell antigen expression [8]. Another study reported a case of CD3-positive plasma cells in PCM infiltrating the liver causing hepatic failure with short survival [20]. In addition, the first case report of the CD3-positive recurrent plasmacytoma also described multiple tumors with recurrence [15]. However, there is no evidence in our case showing disease progression or higher-grade morphology of the tumor cells in the recurrence. Additional studies are necessary to determine the correlation.
The aberrant expression of specific T-cell maker CD3 in B-cell neoplasm is very rare, however, it can be a potential diagnostic pitfall for misclassifying as a T-cell lymphoma [14]. Especially if the neoplastic cells lack expression of common B-cell markers (CD20, CD79a, and PAX5) but are positive for CD3, CD56, and EBER, it can be very challenging to make an accurate diagnosis [14]. To be aware of this rare aberrant expression, more T-cell markers, B-cell markers, and plasma cell markers should be applied in some suspicious and unusual cases. In addition, the molecular analysis for IGH and TRG rearrangements is very helpful in determining the cell origin.
Notes
Ethics Statement
The above case report meets the criteria for Non-Human Subjects Research (NHSR) Self-Determination of University of California Irvine (UCI) IRB. The activities do not constitute human subject research. UCI IRB review is not required. Project activities may begin as soon as the NHSR Self-Determination is submitted to UCI Kuali Research (KRP). The NHSR Self-Determination of this case report has been submitted to UCI KRP. A copy of the submission has been provided to the Journal of Pathology and Translational Medicine. No Protected Health Information (PHI) is included in this case report; therefore, informed consent is waived through a Non-Human Subjects Research (NHSR) Self-Determination of UCI IRB.
Availability of Data and Material
The datasets generated or analyzed during the study are available from the corresponding author on reasonable request.
Code Availability
Not applicable.
Author Contributions
Conceptualization: XZ. Investigation: CN, DR. Supervision: XZ, SR. Writing—original draft: CN. Writing—review & editing: CN, DR, TT, AG, XZ, SR. Approval of final manuscript: all authors.
Conflicts of Interest
The authors declare that they have no potential conflicts of interest to disclose.
Funding Statement
No funding to declare.
