Composite chronic lymphocytic leukemia and mantle cell lymphoma involving the bone marrow: a case report and literature review
Article information
Abstract
Chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL) is a clinically indolent lymphoproliferative disorder characterized by accumulation of mature B-cell lymphocytes. Given the common CD5 co-expression, mantle cell lymphoma (MCL) is one of the most important entities in the differential diagnosis. MCL and CLL/SLL might exhibit overlapping morphologic and immunohistochemical features, making diagnosis particularly difficult in cases of composite lymphomas. Here, we present a unique case of composite lymphoma in an 86-year-old male, along with a literature review on the immunophenotypic variability of both MCL and CLL, which should always be confirmed with additional ancillary cytogenetic and molecular studies.
INTRODUCTION
Composite lymphomas are defined as the simultaneous occurrence of at least two distinct lymphoid neoplasms in the same location. The term was coined by Philip Custer in 1954 and later modified to be applied to the non-Hodgkin’s lymphoma only [1]. The incidence of composite lymphoma is approximately 1.0%–4.7% [2]. Around 20 cases of composite mantle cell lymphoma (MCL) and chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL) have been reported in literature, with the majority being positive for reciprocal chromosomal translocation t(11;14)(q13;q32) or IGH/CCND1 fusion [3-6]. Differentiating between the MCL and CLL/SLL is crucial for clinical management, especially in the presence of an MCL, which needs more aggressive treatment. Morphology and immunophenotype usually represents the first suspicion for composite lymphoma, albeit with its own pitfalls. Cyclin D1, a traditional marker of MCL, has been reported to be positive in the proliferation centers of CLL in 30% of the cases and may be positive in a subset of prolymphocytes [7]. SOX11 is typically expressed in approximately 90% of MCL cases with recent studies showing near 100% specificity and 100% positive predictive value for SOX11 nuclear expression in MCL [8]. SOX11 expression was previously studied in cyclin D1–positive CLL cases and showed to be negative in nearly all cases with only scattered cells in the proliferation centers that could show weak cytoplasmic expression [9].
CASE REPORT
Bone marrow biopsy showed a hypercellular marrow (70%) with sheets of small lymphoma cells arranged in diffuse and nodular pattern (Fig. 1). Half of the lymphoid infiltrate expressed CD5, CD20, CD23, and lymphoid enhancer binding factor 1 (LEF1), suggestive of CLL. The other half of the lymphoid infiltrate expressed CD5, CD20, cyclin D1, and SOX-11, suggestive of involvement by MCL (Fig. 2). The CD20 intensity assessed by immunohistochemistry was found to be similar in both the MCL and CLL areas. p53 was positive on 10%–20% of the lymphoma cells, mainly within the “MCL” area. Ki-67 proliferative index was estimated at 10%. Findings of the flow cytometry are summarized in Table 1. Fluorescence in situ hybridization (FISH) studies performed on fresh bone marrow aspirate for t(11;14) CCND1 and CCND2 rearrangement were negative. However, the loss of one copy of deleted in lymphocytic leukemia (DLEU) locus signals on the long arm (q) of chromosome 13 was detected, a common finding in CLL/SLL. Chromosome analysis showed an interstitial deletion on the chromosome 13q. An in-house next-generation sequencing revealed a missense alteration in TP53, p.I255F. B-cell clonality panel showed detectable clonal IGH and IGK gene rearrangements. Specifically, two polymerase chain reaction (PCR) products were detected in two of the IGH and one of IGK gene rearrangement sites, which may either indicate biclonality or biallelic clonality [10]. Since high suspicion of MCL persisted based on morphologic and immunophenotypic findings, FISH was repeated on formalin-fixed, paraffin-embedded (FFPE) core biopsy tissue and revealed t(11;14)(q13;q32) in 63% of examined nuclei. The final report concluded a diagnosis of composite MCL and CLL. Systemic therapy included Acalabrutinib and Obinutuzumab (6 cycles). A follow-up bone marrow biopsy (36 weeks after initial diagnosis) demonstrated residual/persistent CLL involving 10%–20% of the hypercellular bone marrow in a nodular pattern (Fig. 3). The neoplastic B-cells expressed paired box 5, CD79a, and CD5 with subset positive for CD23 and LEF1 (Fig. 4).
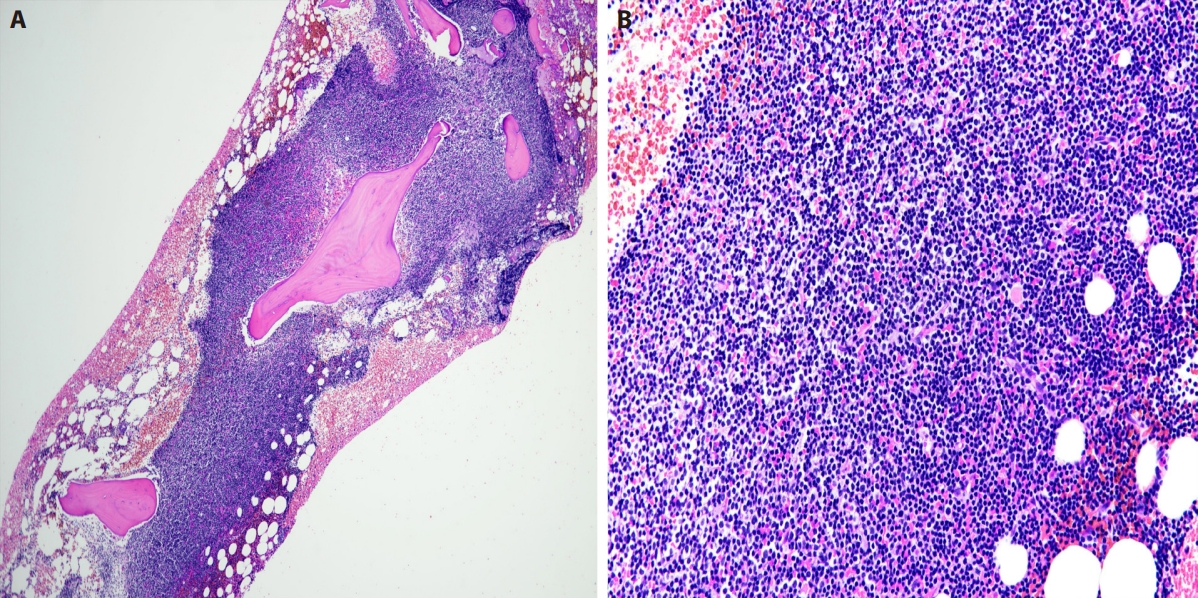
Initial bone marrow. (A) H&E stain, low-power magnification shows extensive involvement by lymphoma. (B) H&E stain, high-power magnification showing the lymphoid infiltrate to be composed predominantly of small cells with scattered interspersed large cells. The cells in the upper half of the infiltrate show slightly more abundant cytoplasm.

A composite picture of immunohistochemical findings from the initial bone marrow. (A) CD20 expression by lymphoma cells with uniform intensity throughout both lymphoid infiltrates. (B) CD5 expression. (C) Cyclin D1 expression, mainly in the upper half of the infiltrate. (D) CD23 expression, more concentrated in the lower half of the infiltrate. (E) SOX11 expression, mainly in the upper half of the infiltrate. (F) Lymphoid enhancer binding factor 1 expression, mainly in the lower half of the infiltrate.
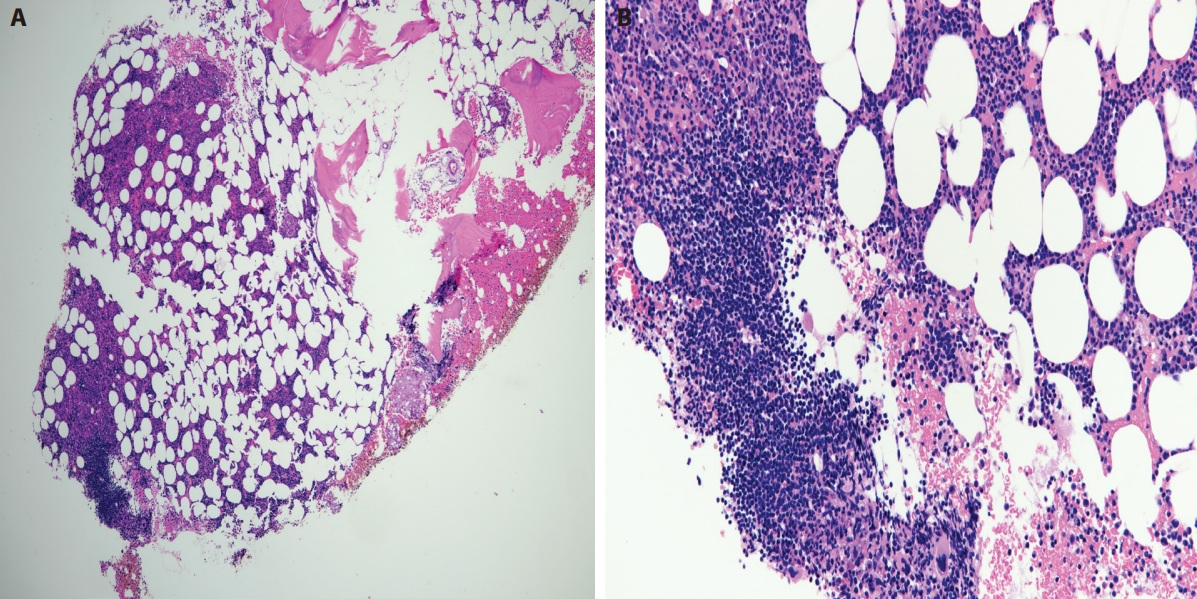
Second bone marrow (post-treatment). (A) H&E stain, low-power magnification shows minor involvement by lymphoma with one small lymphoid aggregate identified. Evidence of trilineage hematopoiesis is noted. (B) H&E stain, high-power magnification showing the lymphoid aggregate to be composed predominantly of small cells.
DISCUSSION
Composite lymphoma refers to the co-occurrence of two clonally distinct populations at the same site, particularly in cases involving low-grade lymphomas. When a combination of low-grade and high-grade component is encountered, it may indicate transformation of the low-grade component. The most described components are MCL and follicular lymphoma, with CLL being less frequent, and cases involving the bone marrow are rare [11]. Our case showed the presence of two distinct populations with an immunophenotype and a distribution pattern suggestive of a composite lymphoma: CLL and MCL. An initially undetected IGH::CCND1 fusion associated with t(11;14)(q13;q32), found in ≥90% of MCL cases, prompted a thorough analysis of the immunohistochemical findings and potential alternative aberrations contributing to the MCL component. Cyclin D1 expression has been reported to be positive in the proliferation centers of CLL in 30% of the cases and it may be positive in a subset of the prolymphocytes [7]. Cyclin D1 upregulation can be related to cryptic rearrangements of IGK or IGL enhancers with CCND1 when CCND1 rearrangement are not found [12]. Some authors suggest that overexpression of cyclin D1 in lymphomas without translocations involving the cyclin D1 gene such as CLL, plasma cell myeloma, or hairy cell leukemia may be due to gene amplification, gene polymorphism, and overexpression induced by oncogenic signals [13]. CCND2 and CCND3 rearrangements may represent other hits in MCL pathogenesis and are present in half of cyclin D1 negative cases [14]. SOX11 is typically expressed in approximately 90% of MCL cases with recent studies showing 100% specificity and 100% positive predictive value for the diagnosis of MCL [8]. Its use in diagnosing other lymphoproliferative disorders is limited; however, cases of SOX11-positive diffuse large B-cell lymphoma, splenic marginal zone lymphoma, Burkitt lymphoma, and B-lymphoblastic leukemia/lymphoma have been reported, suggesting that the gene deregulation is not entirely restricted to MCL [15,16]. Although SOX11 expression was previously reported as negative in all cyclin D1–positive CLL cases [9], other studies reported variable SOX11 staining in the cytoplasm, while the nuclei were completely negative [15,17]. Interestingly, Roisman et al evaluated 86 cases of CLL/SLL using quantitative real-time PCR for SOX11 mRNA expression with 35% of cases showing positive expression. This finding correlated with adverse prognostic factors, such as immunoglobulin heavy chain variable (IGHV) gene mutational status, cytogenetics risk groups, and shorter overall survival [18]. Our findings, including morphology, strong nuclear SOX11 expression, and cyclin D1 positivity in areas not characteristic of proliferation centers, supported composite lymphoma rather than cyclin D1–positive CLL. Additionally, the absence of LEF1 in cyclin D1/SOX 11 positive infiltrates further supported MCL component, given its known positivity in >95% of CLL cases, including the proliferation centers [19]. As a strong suspicion for MCL remained, FISH was repeated on FFPE core biopsy and detected t(11;14). This discrepancy may be explained by the predominant CLL component in the aspirate, which could have hindered the detection of the MCL-associated genetic hallmark. The overall picture supports a diagnosis of composite MCL and CLL.
Notes
Ethics Statement
Informed consent was obtained from an individual participant included in the study. The above case report meets the criteria for Non-Human Subjects Research (NHSR) Self-Determination of University of California Irvine (UCI) IRB. The activities do not constitute human subject research. UCI IRB review is not required. Project activities may begin as soon as the NHSR Self-Determination is submitted to UCI Kuali Research (KRP). The NHSR Self-Determination of this case report has been submitted to UCI KRP. A copy of the submission has been provided to the Journal of Pathology and Translational Medicine. No Protected Health Information (PHI) is included in this case report.
Availability of Data and Material
Data sharing not applicable to this article as no datasets were generated or analyzed during the study.
Code Availability
Not applicable.
Author Contributions
Conceptualization: RD, SAR. Visualization: RD, SAR. Writing—original draft: RD, SAR. Writing—review & editing: all authors. Approval of final manuscript: all authors.
Conflicts of Interest
The authors declare that they have no potential conflicts of interest to disclose.
Funding Statement
No funding to declare.


