Characterization of undifferentiated carcinoma of the salivary gland: clinicopathological and immunohistochemical analyses in comparison with lymphoepithelial carcinoma
Article information
Abstract
Background
This study aimed to reclassify a subset of poorly differentiated salivary gland carcinoma that do not conform to any entities of the current World Health Organization (WHO) classification into the category of undifferentiated carcinoma (UDC) because they lack specific histologic differentiation or immunophenotype.
Methods
Cases of salivary gland carcinomas from Asan Medical Center (2002–2020) that did not fit any existing WHO classification criteria and were diagnosed as poorly differentiated carcinoma, high-grade carcinoma, or UDC, were retrospectively reviewed. Immunohistochemical (IHC) staining for p40, neuroendocrine markers, androgen receptor (AR), and gross cystic disease fluid protein 15 (GCDFP-15) and Epstein-Barr virus (EBV) in situ hybridization (ISH) were performed. Clinical data were collected from the electronic medical records.
Results
Six salivary gland carcinomas did not align with any specific entities and lacked distinct differentiation. Two of six cases displayed lymphoepithelial carcinoma (LEC)-like morphology but were negative or showed negligible immunoreactivity for p40 and EBV ISH, distinguishing them from LEC of the salivary gland. Two cases showed strong AR positivity, suggesting a potential overlap with salivary duct carcinoma (SDC) but lacked classic SDC morphologies and GCDFP-15 expression. No cases expressed neuroendocrine markers.
Conclusions
This study proposes reclassifying these poorly differentiated or high-grade salivary gland carcinomas as UDC based on their indeterminate differentiation and IHC profiles. This may lead to a clearer diagnostic category and enhance our understanding of these high-grade tumors.
INTRODUCTION
Malignant salivary gland neoplasms are rare, constituting approximately 6% of head and neck tumors [1]. They are categorized into unique subgroups based on their distinct histomorphology, immunoprofiles, and molecular alterations [2]. However, a subset of poorly differentiated tumors does not meet the current diagnostic criteria established by the World Health Organization (WHO) classification of head and neck tumors.
Poorly differentiated carcinomas (PDCs) of the salivary glands, which was defined as primary carcinomas showing large and small cell types with or without neuroendocrine differentiation in the 4th edition of the WHO classification of head and neck tumors, were removed in the 5th edition [2,3]. This disease entity included undifferentiated carcinoma (UDC), large-cell neuroendocrine carcinoma, and small-cell neuroendocrine carcinoma.
In recent years, we have encountered several malignant salivary gland tumors that cannot be classified into specific entities based on the WHO classification, prompting a thorough review of our archival cases. The tumor cells of these cases displayed variable histological features; however, some cases were similar to UDC of other head and neck sites and accompanied by abundant lymphoplasmacytic infiltration around the tumor, resembling lymphoepithelial carcinoma (LEC) of the salivary gland. These cases were initially diagnosed as PDC, high-grade carcinoma (HGC), or UDC. This study aimed to redefine these unclassified tumors through a comprehensive investigation of their clinicopathological and immunohistochemical (IHC) features, particularly in comparison with LEC.
MATERIALS AND METHODS
Patient selection
The cases were reviewed and selected from the computerized database of the Asan Medical Center from January 2002 to December 2020, using the search terms “poorly differentiated carcinoma,” “high-grade carcinoma,” “undifferentiated carcinoma,” and “lymphoepithelial carcinoma.” We collected the clinical information of the patients using electronic medical records, including age at diagnosis, sex, tumor location, surgical and adjuvant treatments, postoperative recurrence and metastasis, progression-free survival, and overall survival (OS).
Histopathological analysis
All available hematoxylin and eosin-stained slides were thoroughly reviewed by two board-certified pathologists (S.C. and K.-J.C.). Histopathological data including tumor morphology and architectural patterns, largest dimension of the tumor, extension to extraparenchymal soft tissues, lymphovascular and perineural invasion, metastasis to the lymph nodes, and extranodal extension status were collected. The most representative slides were selected for IHC and in situ hybridization (ISH) studies.
IHC staining
Tissue microarrays were constructed from the formalin-fixed paraffin-embedded blocks for IHC and ISH analysis. Three 2-mm cores of tumor-rich areas were selected from each block. IHC staining was performed using an OptiView DAB IHC Detection Kit on a BenchMark XT automatic immunostaining device (Ventana Medical Systems, Inc., Tucson, AZ, USA) according to the manufacturer’s instructions. After antigen retrieval, the slides were incubated with three antibodies: p40 (1:4, mouse monoclonal, clone BC28, catalog No., Ventana Medical Systems, Inc.), androgen receptor (AR; prediluent, rabbit monocloncal, clone SP107, catalog No. 760-4605, Ventana Medical Systems, Inc.), gross cystic disease fluid protein 15 (GCDFP-15; 1:50, clone 23A3, Neomarkers Inc., Fremont, CA, USA), synaptophysin (1:100, rabbit monoclonal, clone MRQ-40, catalog No. 336R-96, Cell Marque, Rocklin, CA, USA), chromogranin (1:1,600, mouse monoclonal, clone DAK-A3, catalog No. M0869, Dako, Glostrup, Denmark), cytokeratin 7 (CK-7; 1:400, Dako), and p63 (1:200, Dako).
Epstein-Barr virus ISH
Epstein-Barr virus (EBV) detection was performed on paraffin sections using the BenchMark XT automatic immunostaining device (Ventana Medical Systems, Inc.) according to the manufacturer’s instructions, as previously described [4]. Sections were visualized by Ventana EBV ISH iView Blue Detection Kit (catalog No. 800-092, Ventana Medical Systems, Inc.) and INFORM EBV probe (catalog No. 800-2842, Ventana Medical Systems, Inc.). The sections that exhibited strong nuclear signals of the tumor cells were considered positive.
RESULTS
Identification and classification
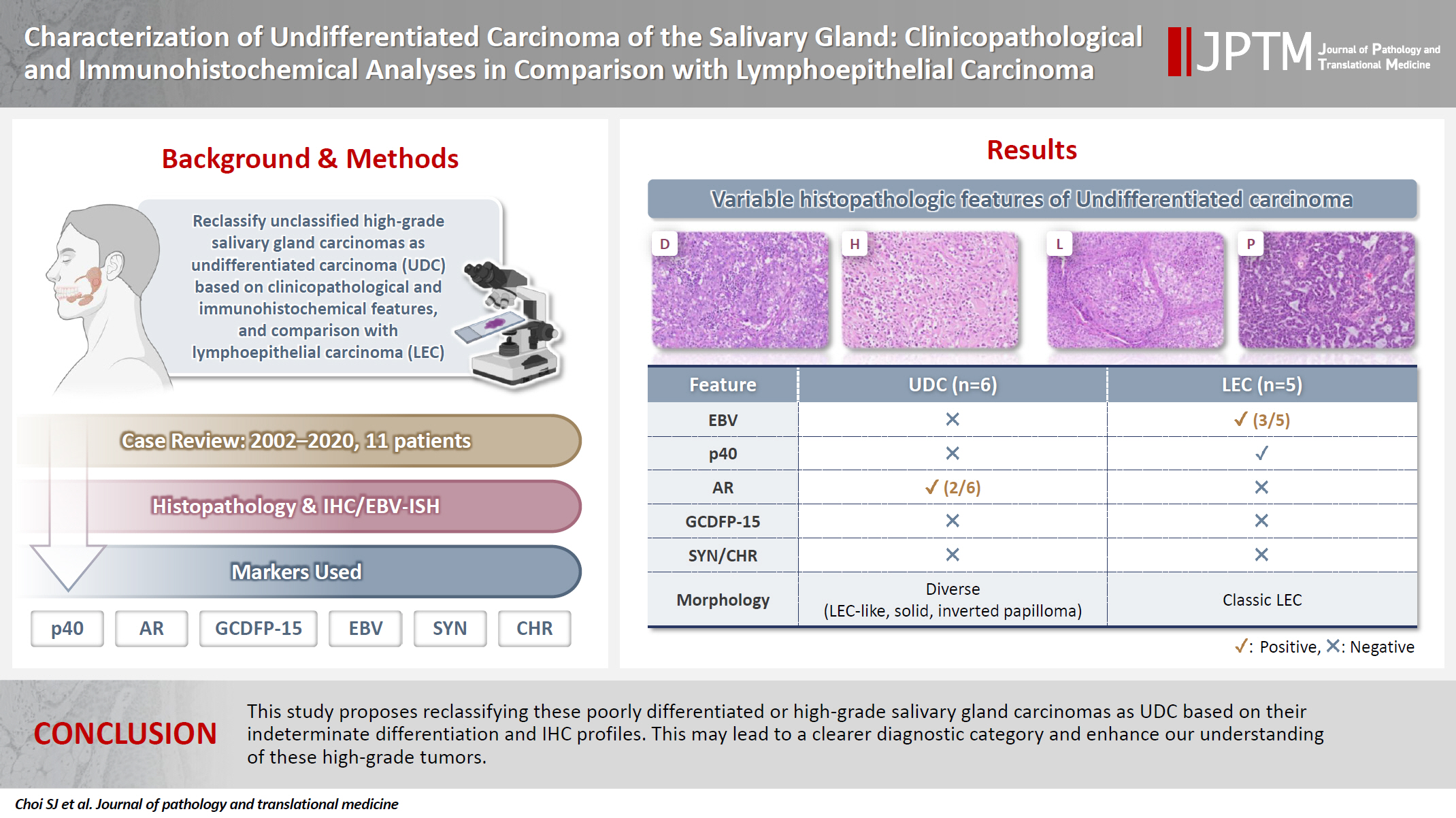
For over 19 years, a total of six patients were diagnosed with PDC, HGC, or UDC of the salivary gland, and five patients were diagnosed with LEC of the salivary gland. The clinicopathological characteristics and IHC and ISH results of the six PDC, HGC, and UDCs and five LEC cases are summarized in Fig. 1. PDC, HGC, and UDCs were distinguished from LEC by negative p40 IHC and EBV ISH.
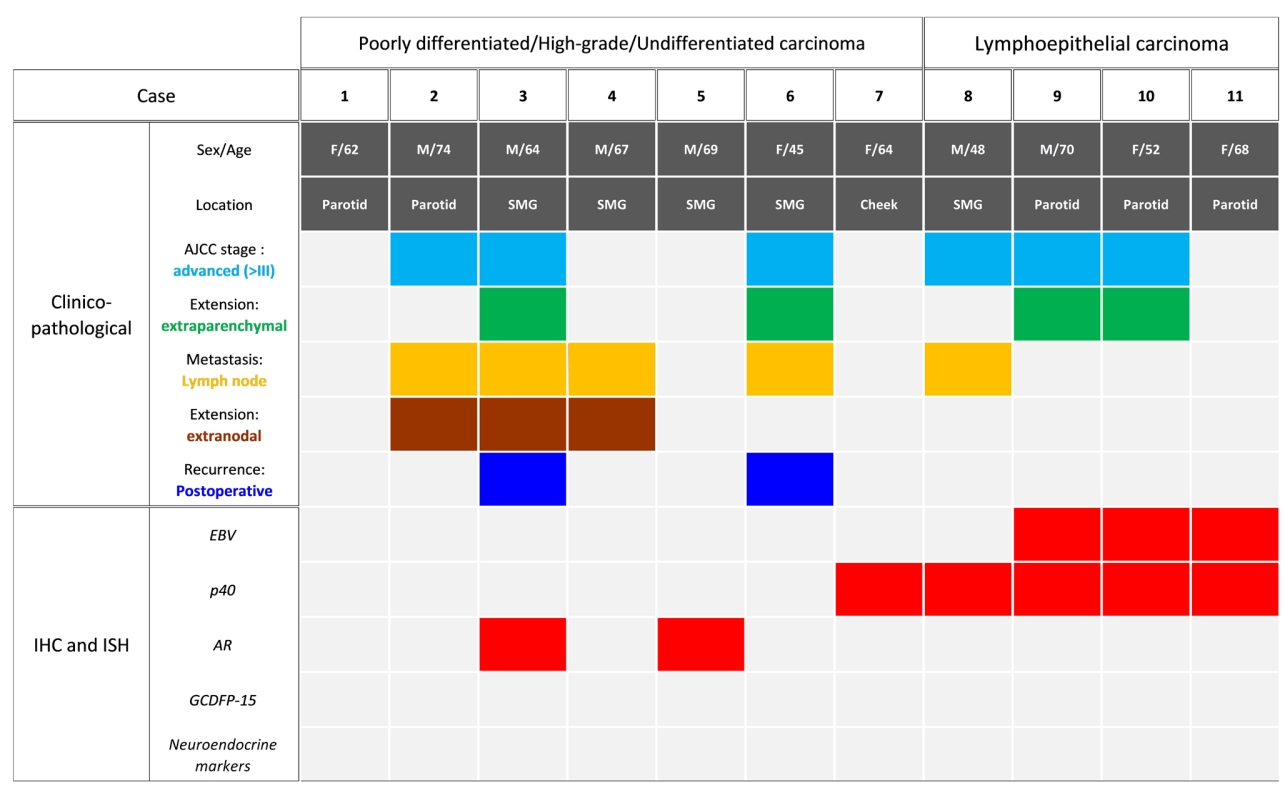
Summary of the clinicopathological and immunohistochemical characteristics of poorly differentiated carcinoma and lymphoepithelial carcinoma of the salivary gland. The gray boxes represent the absence of clinicopathological findings or negative immunostaining, whereas colored boxes (sky blue, green, yellow, brown, blue, and red) represent the presence of clinicopathological findings or positive immunostaining. SMG, submandibular gland; AJCC, American Joint Committee on Cancer; IHC, immunohistochemistry; ISH, in situ hybridization; EBV, Epstein-Barr virus; AR, androgen receptor; GCDFP-15, gross cystic disease fluid protein 15.
Clinical characteristics
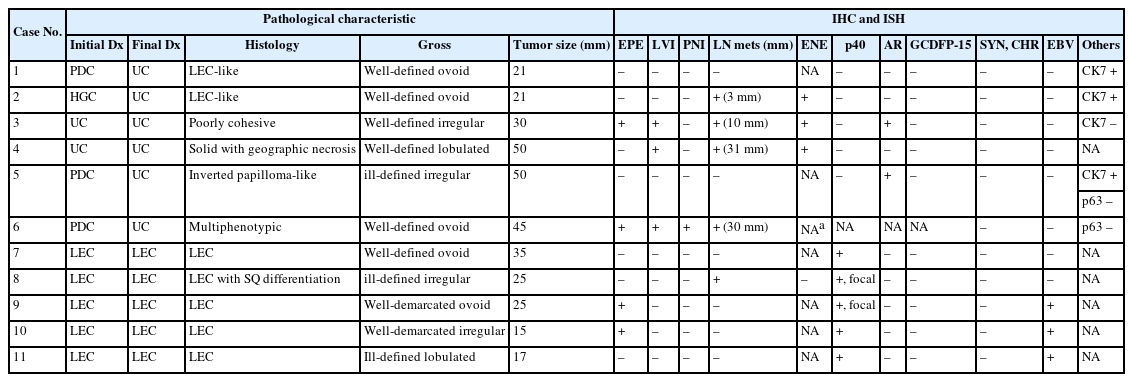
Table 1 summarizes the clinical characteristics of 11 patients. The patients with PDC, HGC, and UDC were between 45 and 74 years old (median, 67 years). Four patients were male, and two were female. Four tumors were located in the submandibular gland, and two were identified in the parotid gland. All patients underwent resection (parotidectomy or wide excision) with neck lymph node dissection. After surgery, three of five patients received adjuvant concurrent chemoradiation therapy and two received radiation therapy. Local or metastatic recurrence developed in the surgical bed or lymph nodes of two patients. The disease-free survival (DFS) ranged from 5 to 139 (mean, 51.8) months, and the OS ranged from 6 to 139 (mean, 54.7) months. Three patients are currently alive, with a mean follow-up period of 84 months.
Patients with LEC were between 48 and 70 years old (median, 64 years), and two were male and three were female. Tumors were located in the parotid gland (3/5), submandibular gland (1/5), and minor salivary gland of cheek (1/5). All patients underwent surgery with or without lymph node dissection. Two patients received postoperative radiation therapy. No patient experienced local tumor recurrence or distant metastasis postoperatively. The DFS and OS ranged from 34 to 258 (mean, 111.2) months. Currently, four patients are alive, and one patient was lost to follow-up.
Pathological characteristics
Table 2 shows pathological characteristics of PDC, HGC, UDC, and LECs. Gross observation of PDC, HGC, and UDCs revealed relatively well-defined, round-to-ovoid, nodular masses. One tumor (case 5) revealed ill-defined, irregular-shaped mass. The cut surfaces of the tumors appeared white or gray-tan and were firm. The greatest dimension of the tumors ranged from 21 to 50 mm (mean, 36.2 mm). Two tumors invaded the extraparenchymal soft tissue. Four cases had neck lymph node metastases with extranodal extension.
In LEC, tumors were grossly well-circumscribed, ovoid, and lobulated, with firm, tan-white cut surfaces. The greatest dimension of the tumors ranged from 17 to 35 (mean, 23.4 mm). Two tumors exhibited extraparenchymal extension. Regional lymph node metastases were found in one patient, with extranodal extension.
PDC, HGC, and UDC cases had diverse histological features (Fig. 2). In cases 1 and 2, tumors displayed LEC-like features, in which variable-sized clusters and sheets of the tumor cells were irregularly infiltrating into the adjacent stroma and arranged in a syncytial pattern without distinct cell borders. The tumor cells were predominantly comprised polygonal or ovoid epithelial cells and possessed abundant clear to eosinophilic cytoplasm and large nuclei with conspicuous nucleoli. Variable amounts of inflammatory cells, particularly lymphoplasmacytic infiltrates present in intratumoral and peritumoral stromal areas. The mitotic activity was increased and easily identified on low-power examination. Keratinization or intercellular bridges of tumor cells indicating squamous differentiation was not observed.
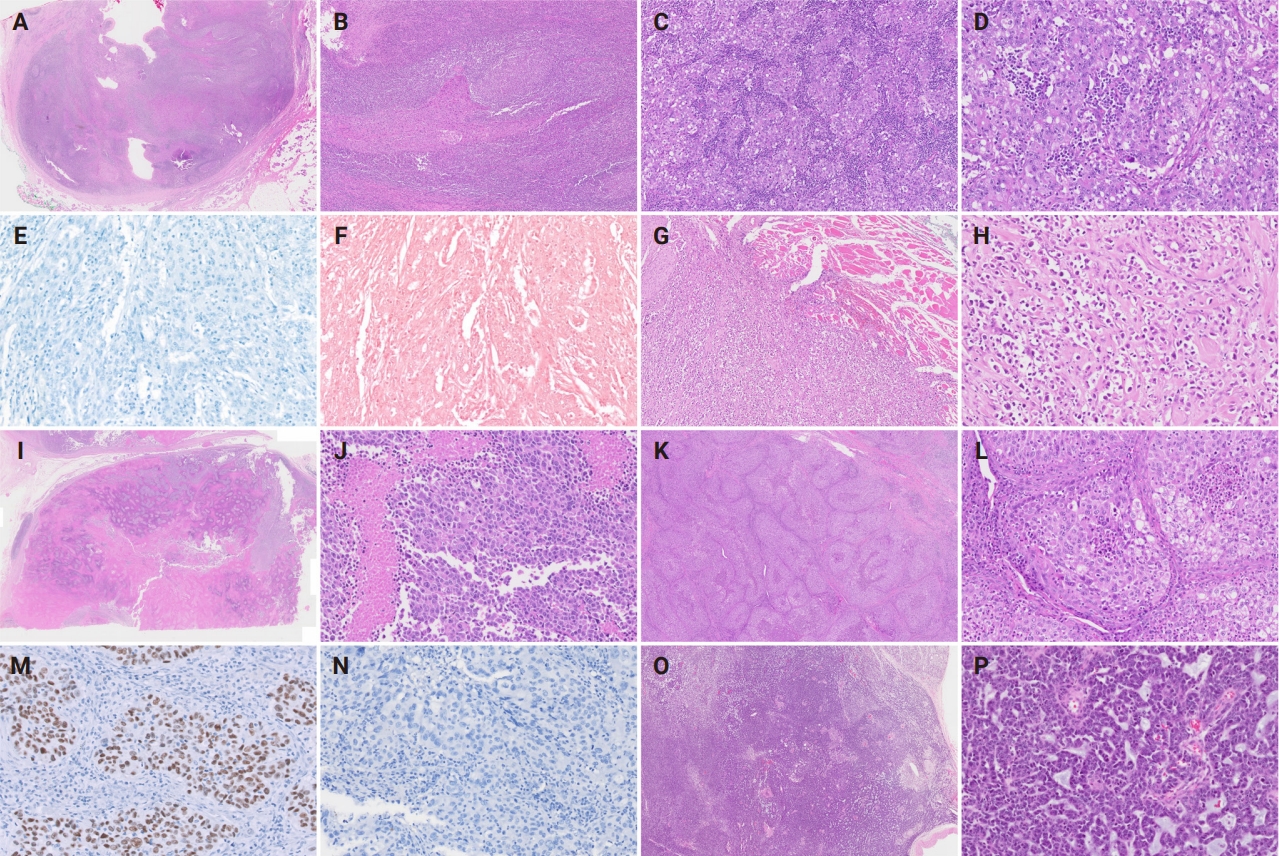
Histopathological findings of poorly differentiated carcinomas with lymphoepithelial carcinoma-like pattern (cases 1 and 2) (A–F), poorly cohesive carcinoma pattern (case 3) (G, H), solid pattern with geographic necrosis (case 4) (I, J), inverted papilloma-like pattern (case 5) (K–N), and multiphenotypic pattern (case 6) (O, P). (A–D) Well encapsulated tumor with abundant lymphoid stroma displays irregular nests and with poorly defined cell borders. (E, F) p40 and Epstein-Barr virus in situ hybridization are negative in undifferentiated carcinoma with lymphoepithelial carcinoma-like feature. (G, H) Poorly cohesive carcinoma cells with no organized pattern shows destructive skeletal muscle invasion. (I, J) Solid sheets of tumor cells with high-grade nuclear atypia are accompanied by distinct massive geographic necrosis. (K, L) Variable-sized nests and sheets of stratified epithelial cells are interanastomosing and possess numerous intraepithelial neutrophils and microabscesses. Tumor cell nuclei are strongly positive for androgen receptor (M) but negative for gross cystic disease fluid protein 15 (N). (O, P) The tumor exhibits mixed architectural patterns including solid, cords, ribbons, and trabecular arrangement. Perivascular rosette-like configurations with myxoid material are easily identified.
In case 3, the tumor was predominantly composed of poorly cohesive cells without any remarkable differentiation. The tumor cells exhibited moderate nuclear pleomorphism with irregular nuclear membrane and hyperchromatic chromatin. Atypical mitosis and tumor necrosis were frequently detected. The degree of stromal lymphoplasmacytic infiltration was lower than those in other PDCs.
Case 4 was characterized by solid sheets of tumor cells with geographic necrosis. The tumor exclusively showed a solid arrangement without other organoid patterns. Large tumor cell necrosis was present with floating islands of tumor nests in variable sizes. The tumor cells possessed abundant eosinophilic cytoplasm with pleomorphic nuclei and showed brisk mitotic activity.
In case 5, inverted papilloma-like histomorphology was observed. Tumor cells consisted of stratified transitional-type epithelium with frequent transmigrating neutrophils and microabscesses, which was reminiscent of sinonasal papilloma of the nasal cavity. The tumor cells displayed vesicular chromatin, prominent cherry-red nucleoli, abundant eosinophilic cytoplasm, and distinct cell borders. The peritumoral intervening stroma was small or narrow and had mild-to-moderate lymphocytic infiltration.
Case 6 was a multiphenotypic HGC with somewhat myoepithelial-cell like histological features. Tumor cells exhibited solid sheets, ill-formed cords, and trabecular or perivascular pseudorosette-like patterns and possessed round nuclei with scanty cytoplasm. Apoptotic bodies and mitotic figures were frequently observed throughout the tumor. The degree of tumor-infiltrating lymphocytes was lower than that of other PDCs or LECs. All cases lacked glandular or squamous differentiation.
The histological features of LEC in the salivary gland were similar to LECs found in other locations (Fig. 3). The tumor cells were arranged in cords, sheets, or nests and harbored moderate amounts of eosinophilic cytoplasm and large vesicular nuclei with prominent nucleoli. Tumor cells had indistinct cell borders, resulting in syncytial growth patterns. Distinct peri- or intratumoral lymphoplasmacytic infiltration and scattered lymphoid follicles were observed. In one case (case 8), tumor cells with occasional keratin pearl formation and dyskeratosis within squamous eddies were identified.
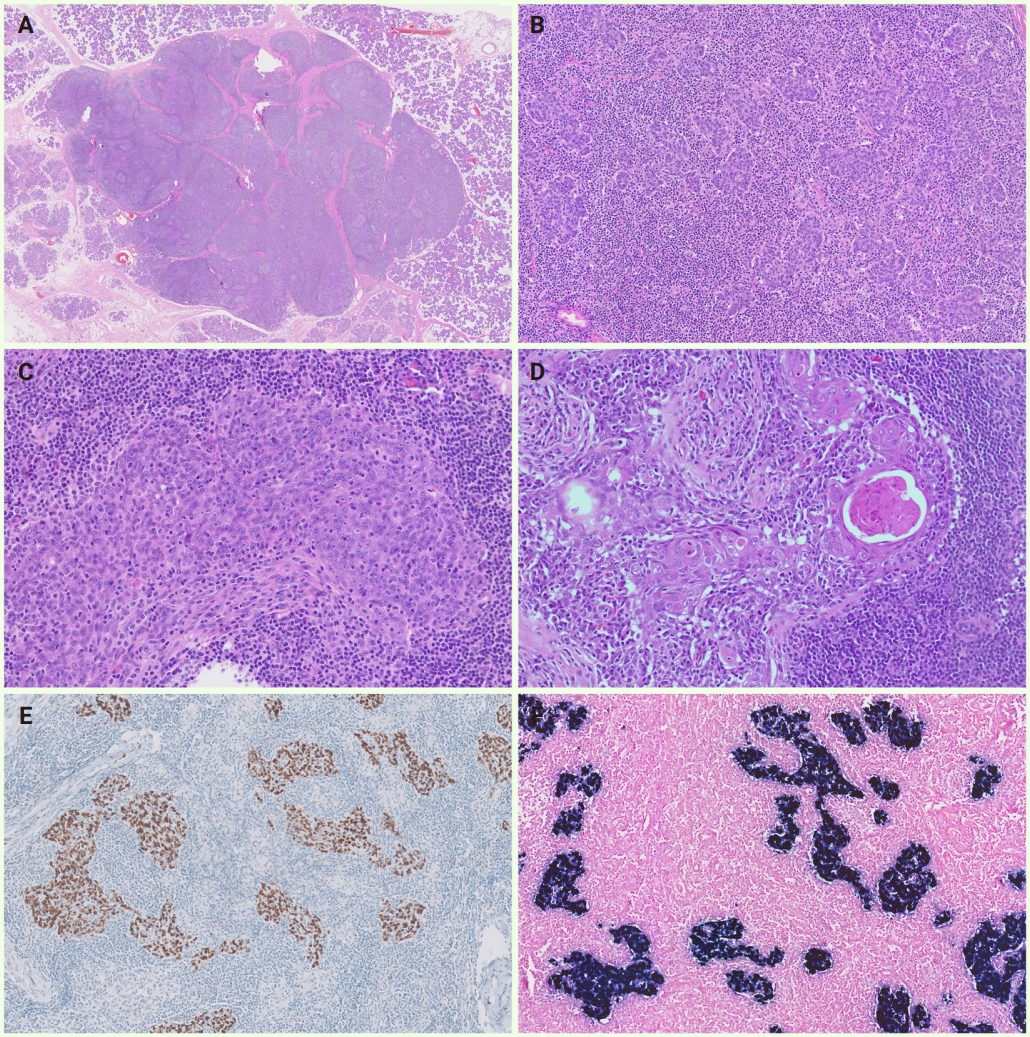
Histopathological findings, immunohistochemical results, and Epstein-Barr virus-encoded RNA in situ hybridization results of lymphoepithelial carcinoma of the salivary gland (cases 7–11). (A) On scanning view, a well-circumscribed nodular mass with fibrous septa is surrounded by the non-neoplastic parotid gland. (B) Low-power magnification shows variable-sized tumor cell clusters infiltrating the stromal tissue with abundant lymphoplasmacytic infiltrates and lymphoid follicles. (C) High-power magnification exhibits an admixture of tumor cells with syncytial appearance and intratumoal inflammatory cells. The tumor cells possessed moderate eosinophilic cytoplasm, large round-to-ovoid nuclei with vesicular chromatin, and single conspicuous eosinophilic nuclei. (D) Squamous differentiation of tumor cells is occasionally present. (E) Tumor cells display strong nuclear p40 expression. (F) Epstein-Barr virus in situ hybridization shows positive signals in tumor nuclei.
Immunostaining and EBV-ISH results
The immunophenotypical characteristics and ISH results are shown in Table 2. PDC, HGC, and UDCs were distinguished from LECs by no or negligible immunoreactivity for p40 and negative EBV ISH. Neuroendocrine differentiation was denied by negative expression for synaptophysin and chromogranin. Cases 3 and 5 displayed diffuse and strong positivity for AR in the tumor nuclei but were negative for GCDFP-15. In case 6, p40, AR, and GCDFP-15 IHCs were unavailable because of insufficient tissue on a paraffin block. Additionally, CK7 and p63 immunostaining results were available in a subset of cases. CK7 was positive in cases 1, 2, and 5, and negative in case 3. Notably, p63 was negative in cases 5 and 6, which may support the absence of squamous or basal/myoepithelial differentiation in these tumors. In contrast, all LECs displayed moderate-to-strong p40 positivity in the tumor nuclei with a variable proportion of staining. Three of the LECs (50%) were diffusely and strongly positive for EBV ISH. Neuroendocrine markers, AR, and GCDFP-15 were all negative in LECs.
DISCUSSION
In the 4th edition of the WHO classification of head and neck tumors, PDC included a subcategory for UDC, which can only be diagnosed in the absence of metastasis from other sites [2,5]. However, clinical features, macroscopic and microscopic findings, or immunohistophenotypes for PDC or UDC have not been described in detail. Moreover, PDC including UDC has been removed in the 5th edition of the WHO classification of head and neck tumors [3].
Nevertheless, certain poorly differentiated (PD) tumors of the salivary gland do not fit into any of the current diagnostic criteria. Thus, classifying these tumors as UDC is more appropriate than simply salivary gland carcinoma not otherwise specified (NOS). Moreover, salivary gland carcinoma NOS in the current WHO classification is described as displaying a wide range of ductal or glandular proliferation patterns, similar to adenocarcinoma NOS, in the previous classification. In this study, UDC was defined as high-grade carcinomas showing no ductal, glandular, squamous, or neuroendocrine differentiation. The six tumors, which were initially diagnosed as PDC, HGC, or UDCs exhibited diverse histologic growth patterns but were devoid of specific differentiation, were classified as UDC. To the best of our knowledge, this is the first study to propose a definition of UDC of the salivary gland, providing a comprehensive analysis of clinicopathological and IHC features of PD tumors of the salivary gland. A diagnostic flowchart summarizing our proposed approach to salivary gland UDC classification is provided in Fig. 4.
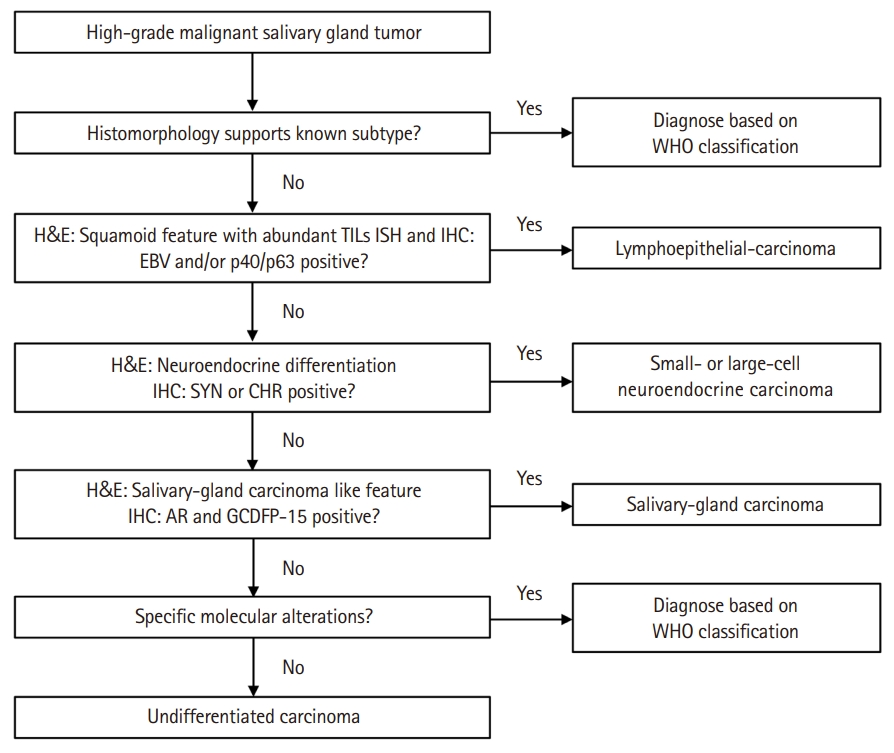
Proposed diagnostic algorithm for undifferentiated carcinoma (UDC) of the salivary gland. The flowchart illustrates a sequential diagnostic approach using histomorphology and immunohistochemistry (IHC) to distinguish UDC from other high-grade salivary gland carcinomas. TIL, tumor-infiltrating lymphocytes; ISH, in situ hybridization; EBV, Epstein-Barr virus; SYN, synaptophysin; CHR, chromogranin; AR, androgen receptor; GCDFP-15, gross cystic disease fluid protein 15; WHO, World Health Organization.
Few studies have documented the clinicopathological and IHC characteristics of UDC of the salivary gland. However, limited IHC and ISH analysis in these studies could not completely exclude other high-grade salivary gland carcinomas and led to an inconclusive classification of the tumors as UDC. Our comprehensive analysis including p40, neuroendocrine markers, AR, and GCDFP-15 IHC and EBV-ISH results, provides a clearer distinction of these PD tumors than other high-grade salivary gland carcinomas including LEC and salivary duct carcinoma (SDC).
Sheen et al. [6] reported the clinicopathological characteristics of 12 patients with salivary gland tumors with undifferentiated morphology. They defined UDC as a malignant epithelial tumor lacking any phenotypic characteristics observable by light microscopy and classified LEC as a distinct UDC subtype. Based on the histology and EBV-ISH results, they classified nine cases as LEC (abundant lymphoid stroma+/EBV+) and three as UDC (lymphoid stroma–/ EBV–). Detailed histological features of UDC cases were not described, and tumor cells only showed positive immunostaining for cytokeratin but were negative for S-100, human melanoma black-45 (HMB-45), leukocyte common antigen, and mucin. Among patients with UDC, one experienced lymph node (LN) metastasis and died within 3 months, whereas another patient without LN metastasis survived for 19 years without recurrence or metastasis.
Hatta et al. [7] reported five cases of UDC of the parotid gland, as well as their clinicopathological features and immunostaining results. They also defined UDC as a malignant epithelial tumor, which is too poorly differentiated to be classified in any other group of carcinoma, and introduced heterogeneous morphologies of tumor cells. The tumors had various histological appearances, including features resembling LEC, epidermoid/mucoepidermoid carcinoma, small cell carcinoma, and malignant hemangiopericytoma. All five cases resulted in death caused by distant metastasis within 2.5 years. IHC analysis for vimentin, smooth muscle actin, desmin, HMB-45, and S-100 was performed to exclude malignant mesenchymal tumors and melanoma, which were negative; however, the analysis did not include squamous or neuroendocrine markers, AR, GCDFP-15, or EBV ISH; thus, classifying the cases as LEC, neuroendocrine carcinoma, SDC, or UDC was inconclusive.
Interestingly, LEC-like features were present in two UDCs (cases 1 and 2) in this study. These tumors require differentiation from true LEC via p40 IHC and EBV-ISH analysis. LECs of the salivary glands exhibit undifferentiated morphology of tumor cells with syncytial sheet-like growth patterns and occasionally have squamous differentiation. Most LECs show diffuse moderate-to-strong p40 or p63 expression on IHCs and are frequently positive for EBV ISH [8,9]. Although the two LEC-like UDC cases in this study were not associated with EBV infection, they lacked histologic and immunophenotypic evidence of squamous differentiation; thus, it could be different from conventional LEC.
In this study, two of the six UDC cases displayed diffuse and strong AR expression, which was frequently reported in SDC [10]. SDC is a primary salivary gland carcinoma showing aggressive biological behavior and has male predilection with old age [11]. Histologically, SDCs demonstrate a complex architecture, including solid, cribriform, and papillary-cystic patterns, often accompanied by comedotype necrosis [12]. The tumor cells are characterized by their large pleomorphic nuclei with coarse chromatin and distinct nucleoli and abundant eosinophilic cytoplasm. Several subtypes including sarcomatioid, mucin-rich, micropapillary, basal-like, oncocytic, and rhabdoid morphology have been reported [13-16]. Cases 3 and 5 had different histological features (poorly cohesive and inverted papilloma-like) but exhibited diffuse and strong AR immunoreactivity, which are possible in high-grade SDCs. However, the lack of the classic morphology of SDC, and the GCDFP-15 expression was not diagnostic for SDC. Moreover, AR expression has been reported in various malignant salivary gland tumors, including primary squamous cell carcinoma, adenoid cystic carcinoma, mucoepidermoid carcinoma, myoepithelial carcinoma, and polymorphous adenocarcinoma [17,18]. Although AR expression is frequently observed in SDC and often supports its diagnosis, it should always be interpreted in conjunction with the tumor’s histomorphologic characteristics and other immunophenotypic features, rather than being used as a standalone diagnostic criterion for SDC.
This study has limitations. First, the clinicopathological results were derived from a relatively small size cohort conducted at a single institution. Given the rarity of UDC of the salivary gland, single-center studies are inherently limited to refine the full clinicopahological spectrum of the disease. Collaborative multicenter studies with larger case numbers, standardized diagnostic protocols, and long-term clinical follow-up are required to enhance the generalizability of findings. Second, one case initially diagnosed as PDC (case 6) was not available for p40, AR, and GCDFP-15 studies, thereby limiting a definitive exclusion of LEC or SDC. Although the tumor demonstrated negative immunoreactivity for p63 and the histological features did not support both entities, the absence of a complete immunophenotypic profile remains a diagnostic limitation. Third, our study did not include a full panel of IHC studies for the differentiation of various salivary gland neoplasms. Given the well-documented morphological overlap among various salivary gland carcinomas, the use of a broader range of immunostainings—including SOX10, DOG1, S100, mammaglobin, and GATA3—is considered essential for accurate classification. In our study, due to the retrospective design and limited availability of archived tissue, we prioritized immunostains based on the predominant histomorphologic features of each tumor. Fourth, molecular testing, such as next-generation sequencing or fluorescence ISH was not performed in this study. Recent literatures highlight the utility of molecular diagnostics in uncovering disease-specific genomic alterations in histologically ambiguous salivary gland tumors. The absence of such ancillary molecular studies in our cohort may have limited our ability to fully exclude specific tumor entities or identify rare genetic subtypes. Future studies should incorporate a more comprehensive IHC panel, along with molecular profiling, to better delineate undifferentiated salivary gland carcinomas.
In summary, this study proposes to elucidate the definition of UDC by comprehensive analysis of histological features through IHC and ISH analyses. We observed several cases of primary high-grade or PDCs of the salivary gland, which are negative for p40, GCDFP-15, neuroendocrine markers, and EBV ISH, so we suggest classifying these tumors as UDC, a category that has been removed in the 5th edition of the WHO classification. Our detailed clinicopathological and IHC analyses of these cases will clarify the categorization of tumors with undifferentiated morphology and enhance our understanding of UDCs in the salivary gland.
Notes
Ethics Statement
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The Institutional Review Board of Asan Medical Center approved this study (IRB No. 2023-1538) and waived informed consent for this study.
Availability of Data and Material
No datasets were generated or analysed during the current study.
Code Availability
Not applicable.
Author Contributions
Conceptualization: KJC. Data curation: SC, GC. Formal analysis: SC. Investigation: SC. Methodology: KJC. Project administration: SC, KJC. Resources: GC, HJL, JSS, YSL, SHC, KJC. Supervision: KJC. Validation: HJL, JSS, KJC. Visualization: SC. Writing—original draft: SC. Writing—review & editing: KJC. Approval of final manuscript: all authors.
Conflicts of Interest
J.S.S., a contributing editor of the Journal of Pathology and Translational Medicine, was not involved in the editorial evaluation or decision to publish this article. All remaining authors have declared no conflicts of interest.
Funding Statement
No funding to declare.