Frozen section histopathology and preanalytical factors affecting nucleic acid integrity in biobanked fresh-frozen human cancer tissues
Article information
Abstract
Background
In this study, we evaluated the effects of storage duration and ischemic time on nucleic acid quality of fresh-frozen tissue (FFT) from colon adenocarcinoma (COAD), hepatocellular carcinoma (HCC), and renal cell carcinoma (RCC) collected at the Cancer Tissue Bank of Seoul National University Hospital.
Methods
A total of 102 FFT samples were analyzed to compare DNA integrity number (DIN) and RNA integrity number (RIN) according to storage duration and ischemic time. Additionally, the effects of histopathologic features—such as tumor cell proportion, inflammatory cell infiltration, and stromal fibrosis—on nucleic acid quality were evaluated.
Results
DIN and RIN remained stable overall even though the storage duration increased, with no statistically significant differences observed. In particular, there was almost no decrease in RNA quality in HCC and RCC samples, but in COAD samples, RIN tended to decrease slightly as the storage duration increased. No significant difference was confirmed between ischemic time and nucleic acid quality, but in COAD tissue, RNA quality variability tended to increase as the ischemic time increased. Furthermore, RIN increased as the tumor cell proportion increased, whereas inflammatory cell infiltration and extracellular mucin pool were identified as independent negative predictors of RIN.
Conclusions
This study confirmed that nucleic acid integrity can be maintained even during long-term storage of FFT and demonstrated that histologic features are closely related to RNA quality. This study would contribute to the establishment of quality assessment and management standards for biobank FFT samples.
INTRODUCTION
Recently, rapid advancements in genome technology and precision medicine have made it increasingly crucial to secure and systematically utilize high-quality biological resources. In particular, the quality of biological samples provided by biobanks determines the reliability and reproducibility of research results, so strict management is required throughout the entire process from sample collection to storage and quality assessment [1-3]. Fresh-frozen tissue (FFT) is preferred for various genomic and transcriptomic studies due to its superior ability to preserve nucleic acid (RNA and DNA) integrity compared to formalin-fixed paraffin-embedded tissue [4,5]. Micke et al. [4] demonstrated that RNA integrity is well preserved in FFT collected as non-fixed surgical specimens, supporting its use in genomic and transcriptomic research [4]. However, the nucleic acid quality of FFT can be sensitively affected by ischemic time, storage duration, and histopathologic features—including tumor cell proportion, combined normal tissue, inflammatory cell infiltration, stromal fibrosis, necrosis, and extracellular mucin pool. Thus, to maximize the utility of FFT, it is essential to specifically and systematically evaluate the effects of these factors on nucleic acid integrity.
Previous studies have been conducted primarily based on samples collected from Western biobanks, and analyses on how ischemic time affects nucleic acid quality across different tissue types have been limited. Except for a study focusing on the effects of fixation and storage conditions on tissue sample quality [6], research on nucleic acid preservation in biobank samples has been rarely conducted in Korea [6]. The studies of Fan et al. [7] and Song et al. [5] reported that RNA quality remains stable in most cancer tissues when ischemic time is within 1 hour, but further research is still needed on the nucleic acid quality change and differences among tissue types according to the ischemic times exceeding 1 hour. In addition, Zhang et al. [8] reported that RNA quality was maintained well even after gastric cancer tissues were stored for up to 13 years, but Neuber et al. [9] reported that RNA quality began to decline after 4.5 years of storage, while DNA quality deteriorated after more than 8 years of storage in tumor tissues derived from the prostate, limbs and trunk, and gastrointerstinal tract. This suggests that the effect of storage duration on nucleic acid quality may vary depending on the tissue type.
To complement the limitations of these previous studies, this study analyzed the effects of sample age (1–10 years) and ischemic time (≤30 minutes, ≤60 minutes, ≤120 minutes, and >120 minutes) on DNA integrity number (DIN) and RNA integrity number (RIN) of FFT from colon adenocarcinoma (COAD), hepatocellular carcinoma (HCC) and renal cell carcinoma (RCC) collected at the Cancer Tissue Bank of Seoul National University Hospital. In addition, the correlations between histopathologic features and nucleic acid quality were analyzed to provide a foundation for establishing quality management and maintenance strategies for FFT in Korean biobanks in the future.
MATERIALS AND METHODS
Collection and storage of FFT
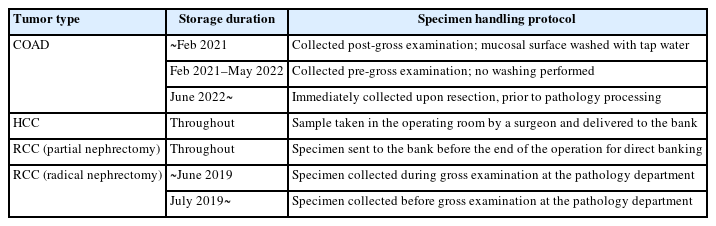
Tissue samples were transported to the laboratory for frozen sections in the operating room immediately after resection and stored at 4°C until collected by the Cancer Tissue Bank. At the bank, the samples were cut into 0.5 × 0.5 × 0.5 cm³ fragments within a range that did not affect diagnosis and placed into 1.8 mL cryovials with anonymous labels. The vials were filled with isopentane (2-methylbutane, >99.0%, Samchun) to fully submerge the tissue, and then immediately stored in a liquid nitrogen tank at –196°C. The liquid nitrogen tank was equipped with a real-time temperature monitoring system, and no thawing occurred for the samples throughout the entire storage duration. However, due to the relocation of the Cancer Tissue Bank laboratory and adjustments in pathology department tasks, some variations existed in long-term and annual tissue collection protocols (Table 1).
This study included a total of 105 FFT samples originating from COAD, HCC, and RCC. The distribution of samples by sample age was 1 year (n = 27), 3 years (n = 25), 5 years (n = 26), and 10 years (n = 27). The distribution of samples by ischemic time was ≤30 minutes (n = 17), ≤60 minutes (n = 18), ≤120 minutes (n = 37), and >120 minutes (n = 33). Samples were selected to ensure a balanced composition of ischemic time and storage duration across tumor types. However, three samples subjected to analysis were excluded from the subsequent analysis since they did not contain any tumor cells as confirmed by hematoxylin and eosin (H&E) staining with frozen sections. Histopathologic features and tapestation-based nucleic acid quality indices for these excluded samples are separately presented in Supplementary Table S1.
Extraction of nucleic acids
Each tissue sample was removed from the liquid nitrogen tank and processed by dividing it into two parts on a sterilized tray. Half of the tissue was stored back in the liquid nitrogen tank (–196°C) for the preparation of frozen sections and H&E staining. The other half was immediately used for nucleic acid extraction.
RNA was extracted using the PureLink RNA Mini Kit (Invitrogen, Thermo Fisher Scientific, Carlsbad, CA, USA). The sample was cut into a size of up to 30 mg using a single-edge blade and placed in a 1.5 mL tube of a BioMasher Ⅱ Grinder (Biofact, Daejeon, Korea). Then, 100 µL of lysis buffer was dispensed and the tissue was homogenized using a pestle. The homogenized tissue was transferred to a new tube, and 900 µL of lysis buffer and 20 µL of 2 M dithiothreitol (DTT) were added, followed by vortexing. RNA was then extracted according to the manufacturer’s protocol. The 2 M DTT solution was prepared by dissolving 0.3085 g of DTT (DTT molecular weight: 154.25) in 1 mL of diethyl pyrocarbonate solution. DNA was extracted using QIAamp DSP DNA Mini Kit (Qiagen, Hilden, Germany) according to the manufacturer’s manual.
RIN was measured using Genomic RNA ScreenTape Kit, RNA ScreenTape Sample Buffer, Ladder of Agilent Tapestation 4200, and Analysis-Software version 5.1 (Agilent Technologies, Santa Clara, CA, USA) [10]. DIN was assessed using Genomic DNA ScreenTape Kit with the same Agilent Tapestation 4200 [10,11].
RIN and DIN of all 105 samples were measured the day after extraction, and the extracted RNA and DNA were stored in a –80°C deep freezer.
Histopathological examination
FFT samples were prepared as frozen section slides. The tissue was placed on the mold of a Cryocut microtome (CM1850UV, Leica Biosystems, Nussloch, Germany), and a small amount of optimal cutting temperature compound was applied before freezing and mounting onto the holder. The sample was trimmed to expose the front surface and then cut into 4 µm-thick sections, which were attached to slides stored at room temperature for fixation.
H&E staining was performed using the quick manual method in the laboratory for frozen sections. After staining with Harris hematoxylin for 1 minute and 30 seconds, the slides were rinsed under running tap water for 10 seconds and dipped 1–2 times in 1% HCl, followed by another rinse under running tap water for 20 seconds until they turned blue. Afterwards, staining was performed with 1% Eosin by dipping them 5–10 times, adjusting the dipping frequency to prevent excessive redness. After staining, they were rinsed under running tap water for 3 seconds, dehydrated by dipping 5–10 times in 95% and 100% alcohol, and finally dipped 10 times in xylene to complete the clearing process.
The stained slides were mounted using synthetic mountant, a non-aqueous mounting medium, with an Automatic Coverslipper (CV5030, Leica Biosystems) to prevent tissue damage and staining degradation.
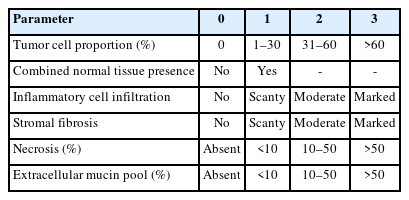
H&E-stained slides of all frozen tissue samples were reviewed by a pathologist (Y.K.), and the suitability of tissue samples was assessed according to the items and criteria established based on ISBER Best Practices (5th edition) [1] and NCI Best Practices for Biospecimen Resources (2016) [2]. Based on the microscopic findings of H&E, various parameters, including tumor cell proportion, combined normal tissue, inflammatory cell infiltration, stromal fibrosis, necrosis, and extracellular mucin pool, were evaluated using a semi-quantitative grading system (Table 2).
Statistical analysis
Statistical analyses were performed using R version 4.4.2 (R Foundation for Statistical Computing, Vienna, Austria). Pearson correlation coefficient and Kendall’s tau (τ) correlation analysis were performed to analyze the correlation between continuous variables. One-way ANOVA and Kruskal-Wallis test were used to compare categorical variables, and nonparametric statistical methods were applied considering the characteristics of the data. For all statistical analyses, two-sided tests were applied, and cases where the p-value was less than 0.05 were considered statistically significant.
RESULTS
Nucleic acid integrity
In this study, nucleic acid integrity was assessed for 102 FFT samples, excluding three samples that did not contain tumor cells. DIN and RIN were measured using the Agilent 4200 TapeStation system.
As a result of the analysis, the mean DIN was 7.20 ± 0.71 with the median of 7.20 (interquartile range [IQR], 6.70 to 7.60), and the mean RIN was 7.03 ± 1.56 with the median of 7.50 (IQR, 6.30 to 8.30). As a result of the quality assessment, the proportion of samples with a DIN of 7.0 or higher was 61.76% (63 out of 102), and the proportion of samples with a RIN of 7.0 or higher was 58.82% (60 out of 102).
Effect of sample age on DNA and RNA integrity
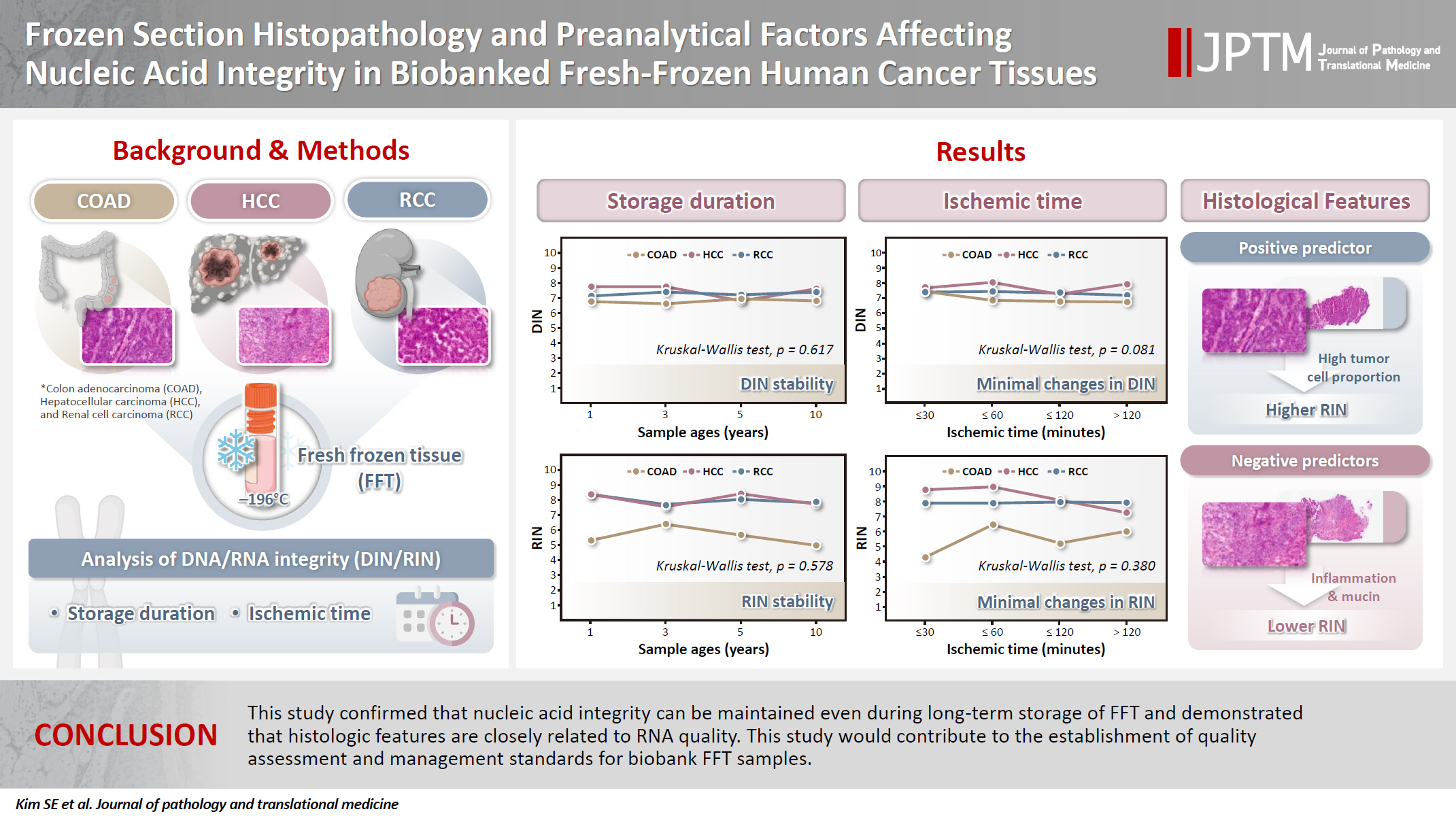
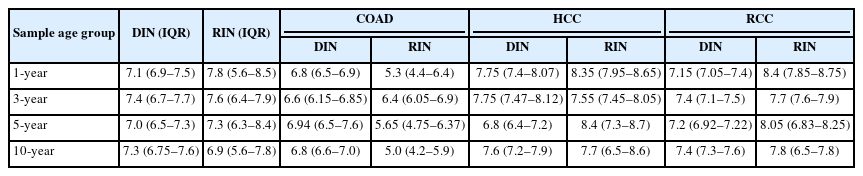
The changes in DIN and RIN according to sample age (1, 3, 5, and 10 years) were compared and analyzed. The DIN showed no statistically significant difference across sample ages (Kruskal-Wallis test, p = .617) (Fig. 1A) and tended to remain stable in all tissues. The median and IQR by tumor type are presented in Table 3. COAD maintained a median DIN level of 6.6 (IQR, 6.15 to 6.85) to 6.94 (IQR, 6.50 to 7.60) despite the increase in sample age, and the variation in DIN according to sample age was not large as well in HCC and RCC, with median DINs of 6.8 (IQR, 6.40 to 7.20) to 7.75 (IQR, 7.47 to 8.12) for HCC and 7.15 (IQR, 7.05 to 7.40) to 7.4 (IQR, 7.30 to 7.60) for RCC, respectively, confirming that DNA integrity remained relatively stable even during long-term storage.

Effect of sample age on DNA and RNA integrity. Each point represents an individual sample. Red circles, blue triangles, and green squares denote samples derived from colon adenocarcinoma (COAD), hepatocellular carcinoma (HCC), and renal cell carcinoma (RCC), respectively. Solid lines connect the median values of each tumor group across the indicated storage durations. (A) Relationship between DNA integrity number (DIN) and sample age. No statistically significant difference was observed among the groups (Kruskal-Wallis test, p = .617). (B) Relationship between RNA integrity number (RIN) and sample age. A similar trend was noted, with no significant difference across storage time (Kruskal-Wallis test, p = 0.578).
Similarly, the RIN showed no statistically significant difference across sample ages (Kruskal-Wallis test, p = .578) (Fig. 1B). However, the change in RIN showed some differences depending on the tumor type. HCC and RCC stably maintained high levels of RIN even as sample age increased, whereas COAD showed a slight decrease from a median of 5.3 (IQR, 4.4 to 6.4) in samples with a sample age of 1 year to a median of 5.0 (IQR, 4.2 to 5.9) in samples with a sample age of 10 years. However, the decrease was minor and did not appear to have a significant experimental meaning due to long-term storage (Table 3).
Effect of ischemic time on DNA and RNA integrity
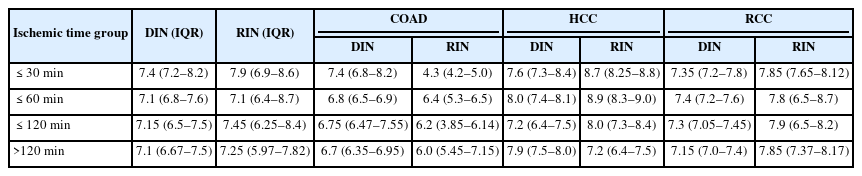
To evaluate the effect of ischemic time on DIN and RIN, samples were divided into four groups for analysis: ≤30 minutes, ≤60 minutes, ≤120 minutes, and >120 minutes. DIN remained relatively constant despite the increase in ischemic time, and no statistically significant differences were observed between the ischemic time groups (Kruskal-Wallis test, p = .081) (Fig. 2A). Similarly, RIN did not show a clear decreasing trend with increasing ischemic time, and the differences between groups were not statistically significant (Kruskal-Wallis test, p = .380) (Fig. 2B).

Effect of ischemic time on DNA and RNA integrity across different organs. Each point represents an individual sample. Red circles, blue triangles, and green squares correspond to samples from the colon adenocarcinoma (COAD), hepatocellular carcinoma (HCC), and renal cell carcinoma (RCC), respectively. Solid lines indicate the median values for each tumor type across ischemic time intervals. (A) Association between DNA integrity number (DIN) and ischemic time (p = .081). (B) Association between RNA integrity number (RIN) and ischemic time (p = .380).
Additional analyses by tissue type revealed that HCC generally maintained the highest DIN and RIN levels. RCC also exhibited relatively stable DIN and RIN despite increasing ischemic time. In contrast, COAD showed a tendency for greater variability in RIN as ischemic time increased, but this tendency was not statistically significant (Table 4).
Histopathologic and preanalytic factors associated with DNA and RNA integrity
In this study, histopathologic features, including tumor cell proportion, combined normal tissue, inflammatory cell infiltration, stromal fibrosis, necrosis, and extracellular mucin pool, were evaluated using a semi-quantitative grading system, and examples of histological evaluations of representative cases observed in COAD, RCC, and HCC are presented in Fig. 3.

Representative histopathologic features of fresh-frozen tissue samples from three different tumor types. Representative histologic features of fresh-frozen tissue samples from colon adenocarcinoma (COAD) (A), hepatocellular carcinoma (HCC) (B), and renal cell carcinoma (RCC) (C). For each tumor type, two cases (left and right) are shown, illustrating the range of histopathologic variation observed. In the COAD samples (A), the left case demonstrates high tumor cell proportion with minimal inflammatory infiltration and mild stromal fibrosis, whereas the right case shows low tumor cellularity with marked inflammatory infiltration and stromal fibrosis. The HCC (B) include a case (left) with high tumor cellularity and minimal background alteration, and a case (right) with lower tumor content and marked inflammatory infiltration and stromal fibrosis. All features were semi-quantitatively graded. The RCC samples (C) show a contrast between a predominantly tumor-rich case (left) with no notable background changes and a case (right) with moderate stromal fibrosis and mild inflammation.
The correlation analysis between histopathologic and preanalytic factors and nucleic acid quality indices (DIN and RIN) is presented in Fig. 4A. No significant correlations were observed between DIN and any of the evaluated variables. In contrast, several significant correlations were identified with RIN. Tumor cell proportion showed a positive correlation with RIN (r = 0.411, p < .001). Inflammatory cell infiltration (r = –0.473, p < .001), stromal fibrosis (r = –0.391, p < .001), extracellular mucin pool (r = –0.255, p = .010), and combined normal tissue (r = –0.223, p = .024) showed significant negative correlations with RIN. Necrosis did not show a significant correlation with RIN (r = –0.148, p = .138).

Univariable and multivariable analysis of factors associated with nucleic acid integrity. (A) Pearson correlation analysis between DNA integrity number (DIN) or RNA integrity number (RIN) and histopathologic or preanalytic variables. Bars represent correlation coefficients (R), and color intensity indicates statistical significance (p-value). (B) Multivariable regression analysis including variables significant in the correlation analysis. Log-transformed RIN was used as the dependent variable. Regression coefficients (β) and 95% confidence intervals (CI) are shown.
To identify independent predictors of RNA integrity, multivariable regression analysis was performed including variables that were significant in the univariable correlation analysis (inflammatory cell infiltration, stromal fibrosis, normal tissue, mucin, and tumor proportion). The results are shown in Fig. 4B. Inflammatory cell infiltration and extracellular mucin pool were identified as independent negative predictors of RNA quality (both p < .05).
DISCUSSION
This study comprehensively analyzed the effects of storage duration, ischemic time, and histopathologic features on DIN and RIN of FFT from COAD, HCC, and RCC collected at the Cancer Tissue Bank of Seoul National University Hospital.
The findings revealed that, despite long-term storage of up to 10 years, most tissues maintained relatively stable levels of both DIN and RIN. HCC and RCC, in particular, demonstrated excellent nucleic acid quality even after more than a decade of storage, confirming that the long-term storage of FFT does not impose practical limitations on its research use.
As a result of analyzing the effects of ex vivo ischemic time on nucleic acid quality (DIN and RIN), no statistically significant decreases in DNA and RNA integrity indices were observed even when ischemic time increased. These results may be because, at the affiliated institution where this study was conducted, surgical samples were stored under refrigerated conditions immediately after resection and until sample processing. Previous studies have also reported that refrigerated storage is effective in suppressing RNA quality deterioration. For example, Fan et al. confirmed that the RIN of COAD decreased rapidly within 8 hours when stored at room temperature, whereas the RIN remained stable for up to 48 hours in refrigerated conditions [7]. In addition, Guo et al. [12] analyzed the relationship between ex vivo ischemic time and RNA quality across various solid cancer tissues, including breast and thyroid cancers, and reported that RNA quality remained stable if ischemic time was kept within 2 hours and immediate cooling was applied, with significant RIN decline occurring only when ischemic time exceeded 4 hours.
However, in the case of COAD, the direction of change was not consistent depending on the ischemic time, and RIN tended to be lower compared to HCC and RCC. This suggests that factors other than ischemic time or sample age may have had a greater impact on RNA quality in the case of COAD, raising the need to find other factors that may have an impact on resource collection and management. In particular, Heumuller-Klug et al. [13] reported that mucosal areas of the COAD are easily exposed to bacteria, leading to rapid RNA degradation and that maintaining cooling and removing bacteria are important for preserving RNA integrity. Although this study did not directly analyze bacteria-related factors, it is highly likely that there are differences in the microbial influence on RNA degradation between the COAD, where gut microbiota normally coexist, and HCC and RCC, which are relatively sterile organs.
In this study, RINs of COAD collected before 2021 (stored for 5 or more years or 10 or more years) were generally lower compared to those collected after 2021. As noted in the methods section, specimens collected before 2021 underwent mucosal surface washing to remove fecal material. Although the direct impact of this process on nucleic acid quality has not been clearly established, previous studies have suggested that excessive exposure to certain washing conditions may affect RNA stability. For example, Ancharayothin et al. emphasized that prolonged washing of intestinal samples can reduce RNA quality and recommended minimizing washing time [14]. These findings suggest that the physical handling of COAD, including the washing step, might have contributed to the lower RINs observed in older samples. Further investigation is needed to clarify the role of such preanalytical factors. These relationships from the univariable analysis are further illustrated in Supplementary Fig. S1, which shows the correlation between histopathologic features and both RIN and DIN.
In this study, we confirmed that histopathologic findings had a greater impact on RNA quality than traditionally recognized factors such as ischemic time and storage age. In univariable analysis, most histopathologic features showed significant associations with RNA quality. Specifically, tumor cell proportion showed a moderate positive correlation with RNA quality, indicating that specimens with a higher proportion of tumor cells generally yielded better RNA integrity. Conversely, inflammatory cell infiltration, stromal fibrosis, extracellular mucin pool, and normal tissue proportion were associated with a decrease in RNA quality. According to the multivariable regression analysis conducted to identify independent predictive factors for RNA quality, inflammatory cell infiltration and extracellular mucin pool were identified as independent negative predictors of RNA quality.
Similar findings have been reported in previous studies. Galissier et al. reported that increased mucin and stromal content led to lower RNA quality in colorectal adenocarcinoma [15]. Several studies have also suggested that severe inflammation can impair RNA preservation. Inflammatory or ulcerated tissues often show increased nuclease activity and disrupted RNA homeostasis. These effects may be mediated by RNase dysregulation [16], tissue acidification [17], or RNase-driven degradation pathways [18]. Giraldo Parra et al. [19] also reported poor RNA recovery from necrotic and inflamed skin lesions. Our findings are consistent with these results. Histopathologic features such as inflammation and mucin content may serve as practical indicators of RNA quality. Incorporating microscopic evaluation may help improve sample selection for transcriptomic studies.
Meanwhile, in the additional examination on the three samples excluded from the analysis in this study as the tumor cell fraction was confirmed to be 0% (Supplementary Table S1), these samples exhibited relatively high DINs despite lacking tumor cells and consisting only of fibrotic tissues or fibrin. Since DIN analysis via Tapestation relies on assessing the fragmentation of high-molecular-weight DNA through electrophoresis, a high DIN may be shown if the DNA is preserved in the nucleus to a certain level or higher. Additionally, DNA can remain relatively stable even within necrotic or fibrotic tissues, potentially leading to misinterpretation of DNA quality. Therefore, using Tapestation-based DINs as the sole criterion for sample suitability may allow the inclusion of tissues that are actually inappropriate for analysis. Accordingly, it is desirable to conduct a histological evaluation of the frozen section through HE staining in parallel with nucleic acid analysis using FFT.
This study has several limitations. First, the tissue samples used for the analysis were limited to samples collected from a single institution, the Cancer Tissue Bank of Seoul National University Hospital, and the total number of samples (n) was relatively small, which may limit statistical interpretation. Second, this study included only three types of cancer tissues (COAD, HCC, and RCC), without including other cancer types or non-neoplastic tissues, which may limit the generalizability of the findings across broader sample types. If large-scale, multi-institutional retrospective studies are conducted in the future—including various tumor and non-tumor tissues collected under diverse conditions—the generalizability and reproducibility of the findings could be further enhanced.
In conclusion, this study confirmed that the storage duration and ischemic time of FFT had generally limited effects on DNA and RNA quality. In particular, COAD showed a tendency for RNA quality to deteriorate relatively easily, suggesting that more stringent preprocessing and quality control are necessary for the tissue. Additionally, inflammatory cell infiltration and extracellular mucin pool were identified as independent predictors of RNA degradation, highlighting the importance of considering histopathologic information as a key factor in the quality assessment of biobank samples. This study is significant in that it comprehensively analyzed the preprocessing conditions and histological factors affecting nucleic acid quality in various cancer tissues collected from a real clinical setting. In the future, the findings may serve as a practical and foundational resource for improving biobank quality management guidelines and establishing a high-quality molecular research infrastructure. Furthermore, our findings are consistent with the recent recommendation by Kim et al. [20], which emphasizes the urgent need for systematic education and training programs aligned with the ISO 20387 international standard. In particular, the identification of inflammatory cell infiltration and extracellular mucin pool as independent predictors of RNA degradation highlights the importance of strengthening biobank personnel training to ensure that high-quality biospecimens can be selected and managed based on histological quality criteria. Such an approach may help enhance the overall quality control of biobank operations and contribute to the establishment and advancement of ISO 20387-compliant biobanking systems.
Supplementary Information
The Data Supplement is available with this article at https://doi.org/10.4132/jptm.2025.07.22.
Notes
Ethics Statement
Tissue samples used in this study were COAD, HCC, and RCC samples collected at the Cancer Tissue Bank of Seoul National University Hospital from January 2014 to December 2023. The Cancer Tissue Bank of Seoul National University Hospital collects and stores human-derived biological samples with the approval of the Institutional Review Board (IRB) of the affiliated institution, and all tissue samples were collected after obtaining voluntary written consent from the donor or legal representative in accordance with the Declaration of Helsinki. This study was conducted as part of the quality assurance process regularly performed by the biobank, and the purpose and method of the study met the review exemption criteria, so approval for review exemption was obtained from the IRB of Seoul National University Hospital (IRB No. E-2503-168-1625).
Availability of Data and Material
The datasets generated or analyzed during the study are available from the corresponding author on reasonable request.
Code Availability
Not applicable.
Author Contributions
Conceptualization: YK, HSL. Data curation: SK, JK. Formal analysis: SK, BK. Investigation: SK, BK, JK. Funding acquisition: HSL. Methodology: YK, HSL. Project administration: HSL. Resources: HSL. Software: SK. Supervision: YK, HSL. Visualization: SK. Writing—original draft: SK. Writing—review & editing: BK, YK, HSL. Approval of final manuscript: all authors.
Conflicts of Interest
The authors declare that they have no potential conflicts of interest.
Funding Statement
This research was supported by the National Research Foundation of Korea (NRF) grant funded by the Korean government (Ministry of Science and ICT) (No. RS-2024-00337984)