Invasive Cribriform Carcinoma Arising in Malignant Phyllodes Tumor of Breast: A Case Report
Article information
Abstract
Phyllodes tumor is an uncommon fibroepithelial neoplasm of the breast. And it is characterized by expanded stroma with increased cellularity and elongated epithelium-lined clefts. Mammary carcinomas within phyllodes tumors have been rarely reported. To date, however, no reports have described the invasive cribriform carcinoma arising in malignant phyllodes tumor. Here, we report a 62-year-old woman who presented with a large breast mass. Microscopically, the mass was a typical malignant phyllodes tumor showing well developed leaf-like architecture and stromal overgrowth with high cellularity and nuclear pleomorphism. In a portion of the tumor, however, the epithelial component showed a cribriform pattern of proliferation in the absence of myoepithelial cells, suggestive of the invasive cribriform carcinoma. To our knowledge, this is rare and it is difficult to make a differential diagnosis of it. Here, we report our case with a review of literatures.
Phyllodes tumor is a rare fibroepithelial neoplasm that accounts for less than 1% of all breast tumors,1 and is characterized by expanded stroma with increased, often varying cellularity and mitotic activity. In addition, elongated epithelium-lined clefts resembling leaf-like elements in the absence of a uniformly hypocellular stroma are suggestive of phyllodes tumor.2 No single feature is reliable in predicting its clinical behavior. Several grading systems of the phyllodes tumor have been proposed, none of which has been universally agreed upon. Larger size of the phyllodes tumor is more likely to be malignant, but assessment of its biologic behavior is based on histologic features including stromal cellularity, atypia, mitoses, infiltrative border, stromal overgrowth, and the presence or absence of necrosis.1,2
Development of mammary carcinoma within a phyllodes tumor is exceedingly rare. Only a few cases of in situ or invasive carcinomas within malignant phyllodes tumors have been previously described.3-8 Here we report a case of invasive cribriform carcinoma arising in a malignant phyllodes tumor in Korea.
CASE REPORT
A 62-year-old woman visited Seoul National University Bundang Hospital due to a palpable mass in the left breast. The patient had a 15-year history of a painless mass of 3 cm in size which was increased over a period of six months. The patient had no family history of breast cancer or that of other medical conditions. Sonographic examination revealed an approximately 10 cm sized lobulated mass with septa inside. Core needle biopsy revealed a fibroepithelial lesion with a mild increase in the stromal cellularity. The patient was operated by simple mastectomy without lymph node dissection under a provisional diagnosis of benign phyllodes tumor. The surgical specimen was measured as 16.5×16.0×8.0 cm. Grossly, the excised mass involved the nipple, measuring 10.0×9.5×9.0 cm, and it had a relatively ill-defined margin. The cut surface was whitish-yellow in color and it was lobulated with variably fleshy and firm areas (Fig. 1). Histologically, most of the tumor displayed typical characteristics of phyllodes tumor and these included tumor necrosis, the stroma with moderate-to-high cellularity, stromal overgrowth, moderate to severe nuclear atypia and increased mitotic activity (6 mitotic figures per 10 high power fields). These characteristics met the World Health Organization (WHO) criteria for a malignant phyllodes tumor (Fig. 2A-C). In a part of the tumor, however, epithelial components were replaced by invasive carcinoma (Fig. 2D). It was 6.0 cm in its greatest dimension and mainly showed a cribriform pattern of growth with well-formed tubular structures (Fig. 3A) with low nuclear grade and low mitotic activity (1 mitotic figure per 10 high power fields). No ductal carcinoma in situ component was found.
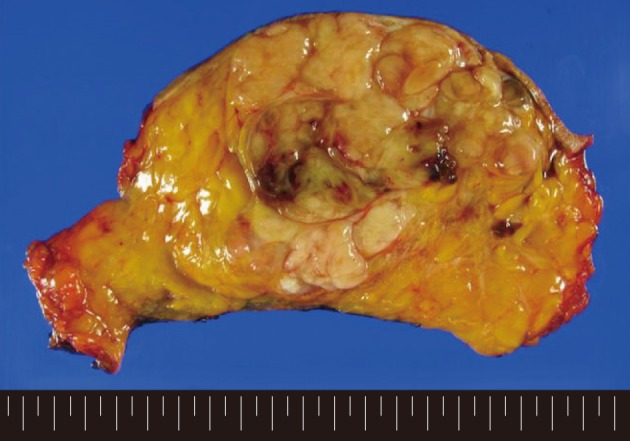
Gross findings of the tumor. The cut surface is whitish-yellow in color and lobulated with variably fleshy and firm areas. The tumor has a relatively ill-defined margin.
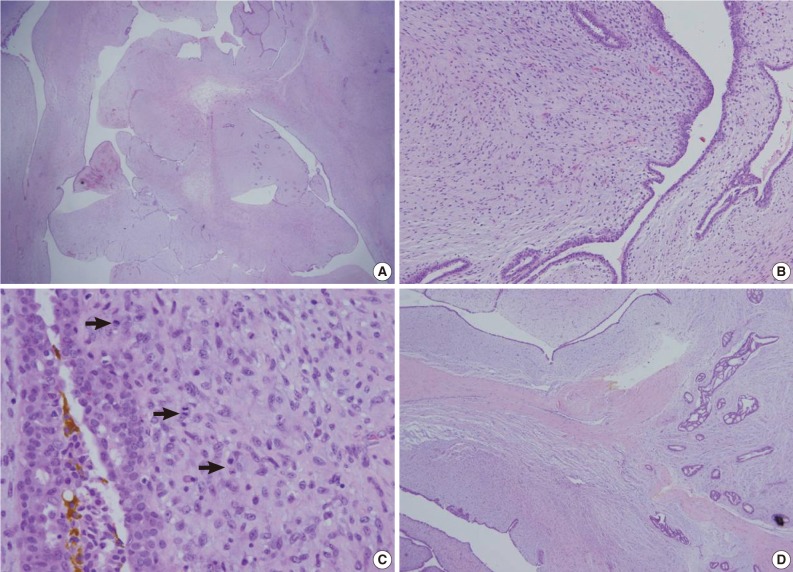
Histopathologic findings of the tumor. (A) Expanded stroma with increased cellularity and elongated epithelium-lined clefts resembling leaf-like elements are suggestive of phyllodes tumor. (B) Higher magnification reveals increased stromal cellularity and nuclear atypism. (C) Mitotic figures are also frequently found (arrows). (D) On the right side of the section, epithelial component shows a cribriform pattern of proliferation, which is consistent with the findings of the invasive cribriform carcinoma.
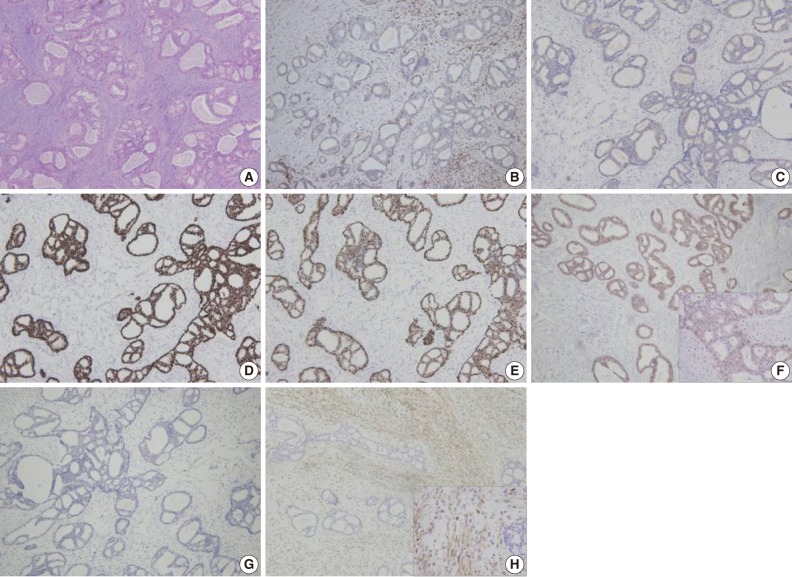
(A) Representative section of the invasive cribriform carcinoma. (B-H) Immunohistochemical findings of the tumor. The tumor reveals a lack of the myoepithelial cells by calponin (B) and p63 (C) staining. The tumor is immunoreactive for estrogen receptor (D) and progesterone receptor (E) but negative (1+/3) for human epidermal growth factor receptor 2 (F). The tumor cells are negative for cytokeratin 5/6 (G) and epidermal growth factor receptor (H). However, stromal cells are reactive for epidermal growth factor receptor.
Immunohistochemical staining of p63 and calponin demonstrated a lack of the myoepithelial cells within the area of invasive carcinoma (Fig. 3B, C). The tumor cells were immunoreactive for estrogen receptor (100%) and progesterone receptor (95%) and negative for human epidermal growth factor receptor 2 (HER-2/neu) (1+/3) (Fig. 3D-F). In addition, they showed no immunoreactivity for cytokeratin 5/6 or epidermal growth factor receptor (EGFR) (Fig. 3G, H). However, stromal cells forming the malignant phyllodes tumor were immunoreactive for EGFR.
One month later, the patient underwent left axillary lymph node dissection. Subsequently, the pathological examination revealed no nodal metastasis. The patient received adjuvant fluorouracil, adriamycin, and cyclophosphamide chemotherapy for invasive cribriform carcinoma. Almost two years postoperatively, the patient survived with a disease-free status.
DISCUSSION
Phyllodes tumor is an uncommon biphasic breast tumor and it constitutes 1% of all breast tumors and 2-3% of fibroepithelial neoplasm.2 Its histologic features include stromal hypercellularity and long clefts lined with several layers of epithelium. Despite a lack of the standard grading system, phyllodes tumors are traditionally graded into benign, borderline, and malignant.1,2 According to three tiered grading subgroups of the WHO classification, a benign phyllodes tumor has modest degree of the stromal hypercellularity, little cellular pleomorphism, few mitotic figures, well circumscribed margin and uniform stromal distribution. On the other hand, a malignant phyllodes tumor shows marked stromal hypercellularity, marked cellular pleomorphism, numerous mitotic figures (>10 /10 high power fields), an infiltrative margin and stromal overgrowth. A phyllodes tumor which displays indeterminate features is categorized as a borderline malignancy.9 All phyllodes tumors may recur, but only the borderline and malignant phyllodes tumors metastasize.10 Although the mitotic activity was modest (6/10 high power fields) in our case, the tumor had stromal overgrowth, marked stromal hypercellularity and marked cellular pleomorphism. These findings are suggestive of the malignant phyllodes tumor.
The epithelial component of phyllodes tumor may show some proliferative changes. However, its malignant changes within phyllodes tumor are very rare. To date, only several cases of lobular carcinoma in situ,8 invasive lobular carcinoma,11 ductal carcinoma in situ,3,4,6,7,12 invasive ductal carcinoma,4,5,13-15 ductal and lobular carcinoma in situ16 and squamous carcinoma13,17 in phyllodes tumors have been reported.
First described by Page et al.18 in 1983, invasive cribriform carcinoma is very rare and it is a unique type of the breast carcinoma. The invasive cribriform carcinoma is defined as the invasive carcinoma of breast showing more than 50% of cribriform pattern in the invasive component. Page et al.18 divided it into the classical and mixed invasive cribriform carcinoma. The classical invasive cribriform carcinoma exclusively shows cribriform pattern or mainly cribriform pattern with a limited extent of tubular structure, while mixed one contains the areas of less well-differentiated invasive carcinoma. In our case, the invasive carcinoma component showed the characteristic features of invasive cribriform carcinoma, i.e., a cribriform pattern of the growth of tumor cell nests and a few well-formed tubules with mild nuclear atypia. According to criteria of Page et al.,18 our case can be diagnosed as a classical invasive cribriform carcinoma arising in a malignant phyllodes tumor. In our case, however, the cribriform nests were not aggregated. This is in contrast to the classic invasive cribriform carcinoma arising in normal breast. They were widely dispersed by the expanded stroma in some areas, thus making the differential diagnosis difficult. The cribriform pattern of tumor cells may also be present in ductal carcinoma in situ, adenoid cystic carcinoma and secretory carcinoma, all of which should also be considered in the differential diagnosis. Ductal carcinoma in situ typically has myoepithelial component. In our case, however, immunohistochemical staining of p63 and calponin revealed no myoepithelial cell layers. Adenoid cystic carcinoma also may exhibit cribriform patterns. And it has biphasic cellular components of the basaloid cells and ductal epithelial cells. Besides, it is negative for estrogen receptor, progesterone receptor and HER-2/neu.19 In the current case, there were no basaloid cells and basement membrane-like material in the lumen, both of which are the histopathologic findings of the adenoid cystic carcinoma, and there was a positive response to estrogen receptor and progesterone receptor by immunohistochemistry. Secretory carcinoma has similar characteristics to lactating mammary glands and it is characterized by extensive secretions.20 However, these findings were not seen in our case.
A majority of previous reports have shown that patients with carcinoma in phyllodes tumor achieve a good prognosis. To date, however, there are no methodical studies about or consensus on the prognosis of the above cases. Of a total of 13 cases that have been reported up to present, 11 were followed up to monitor a clinical course. Nine patients survived with no evidence of disease progression during the follow-up period ranging from three months to nine years,3,4,6,8,11,12,14,15,17 and one patient died of the unrelated disease after a 9-year follow-up observation with no recurrence or metastasis.7 However, one patient experienced tumor recurrence. Sugie et al.5 reported a case of 54-year-old woman who had invasive ductal carcinoma with squamous differentiation arising in malignant phyllodes tumor. Two years and eight months postoperatively, this patient experienced lung and facial bone metastases of the sarcomatous component. Approximately 22% of patients with malignant phyllodes tumors develop distant metastases where the lung and bone are affected most frequently and these patients eventually die of disease.9 In our case, it is difficult to predict the prognosis. The patient was followed up for short-term period and there were no recurrence or metastasis.
In conclusion, our case is the first report of invasive cribriform carcinoma arising within malignant phyllodes tumor. Although carcinoma arising in phyllodes tumor is rare, it should be considered in the diagnosis of phyllodes tumors with abnormal epithelial proliferation.
Notes
No potential conflict of interest relevant to this article was reported.