Frequency of BRAF Mutation and Clinical Relevance for Primary Melanomas
Article information
Abstract
Background
This study was conducted to clarify the frequency of the BRAF mutation in primary melanomas and its correlation with clinicopathologic parameters.
Methods
We analyzed the frequency of BRAF mutation in patients with primary cutaneous melanoma (n=58) or non-cutaneous one (n=27) by performing dual priming oligonucleotide-based multiplex real-time polymerase chain reaction to isolate and to purify the DNA from the formalin-fixed and paraffin-embedded tumors.
Results
The BRAF mutation was found in 17.2% (10/58) of patients with primary cutaneous melanoma and 11.1% (3/27) of those with non-cutaneous melanoma. The frequency of BRAF mutation was not correlated with any clinicopathologic parameters with the exception of the patient age. The frequency of the BRAF mutation was significantly higher in patients younger than 60 years as compared with those older than 60 years (p=0.005).
Conclusions
Compared with previous reports, our results showed that the frequency of the BRAF mutation was relatively lower in patients with primary cutaneous melanoma. Besides, our results also showed that the frequency of the BRAF mutation had an inverse correlation with the age. Further studies are warranted to exclude methodological bias, to elucidate the difference in the frequency of the BRAF mutation from the previous reports from a Caucasian population and to provide an improved understanding of the molecular pathogenesis of malignant melanoma.
In Korea, the incidence of malignant melanoma (MM) among dermatology outpatients has been reported to be 0.02%.1 In addition, it has been observed that patients who are diagnosed with MM account for 7.1% of total patients with malignant skin tumor.1 This proportion is relatively lower as compared with a Caucasian population. The MM remains a highly aggressive tumor. Patients with advanced MM are refractory to the medical treatments that are currently available in a clinical setting. In addition, these patients commonly have a very poor prognosis, with a median survival period of six months.2 To date, considerable efforts have been made to discover treatment modalities that are effective for the management of advanced MM. Recent progress in understanding the molecular pathogenesis of MM has facilitated the development of targeted drugs and new therapeutic approaches for it.3
The BRAF somatic mutation is one of the well-established molecular abnormalities contributing to the pathogenesis of MM. It was first reported to have a relationship with MM in 2002. In more than 90% of total BRAF mutations, a glutamic acid for valine substitution at codon 600 in exon 15 has been identified up to present.4 This genetic alteration of BRAF sequentially induces constitutive extracellular signal-regulated kinase (ERK) signaling through a hyperactivation of the RAS/RAF/mitogen-activated protein kinase/ERK (MAPK/ERK) pathway that is involved in promoting the proliferation, survival and development of tumor cells.
To date, several studies have been conducted to examine the relationship of BRAF mutation in MM with its clinicopathologic characteristics. But, most of these studies have been conducted in the Caucasian population. There are only a limited number of reports in the Asian population. MM presents a variety of clinical and histological features between the ethnic populations. Nevertheless, to our knowledge, there are no reports about the associations between BRAF mutations in MM and its clinicopathologic features in the Korean population.
Given the above background, we conducted this study to examine the frequency and potential clinicopathologic significance of the BRAF mutation in Korean patients with primary cutaneous or non-cutaneous melanoma.
MATERIALS AND METHODS
Patients
We retrieved the pathology data of 58 patients with cutaneous primary melanoma (n=58) and 27 non-cutaneous one (n= 27) from the archives of surgical pathology at Dong-A University Hospital and Samsung Changwon Hospital during a period ranging from 1997 to 2008. All the pathological slides were thoroughly reviewed by two pathologists in a blind setting. Through a retrospective analysis, we collected demographic and clinical data such as the age, sex, the site of tumor occurrence, histological subtype, Breslow thickness, ulceration, nodal metastasis, distant metastasis, the American Joint Committee on Cancer (AJCC) clinical stage,5 the recurrence of disease after the initial diagnosis and survival. The follow up persisted until September 2011 or until the death of patients or loss of follow up with the patients. The current study was approved by the Institutional Review Board of our medical institution (10-10-188).
Tumor samples
We obtained 85 tumor specimens which were formalin fixed and paraffin embedded following a surgical excision biopsy that had been. We sectioned tumor specimens at a thickness of 10-µm using a sterile microtome blade and thereby prepared two tissue samples. We repeated to prepare tissue samples three times, thus ensuring the reproducibility.
DNA extraction
Genomic DNA (gDNA) was extracted from two (10 µm thickness) slices of formalin-fixed paraffin-embedded (FFPE) material using the QIAamp DNA FFPE extraction kit and the QIAcube automated DNA extraction machine (Qiagen, Hilden, Germany). This was quantification by UV absorption (Nanodrop, Thermo Scientific, Wilmington, DE, USA), typically yielding a total of >1 µg of gDNA per specimen. We performed all the experimental procedures according to the manufacturer's protocol.
Mutation analysis
The BRAF mutation was detected using the Anyplex BRAF V600E real time detection system (Seegene Inc., Seoul, Korea). The reaction mixture contained a 2 µL of 10× BRAF Oligo Mix (OM) including amplification and detection reagents, a 3 µL of 8-methoxypsoralen (8-Mop) solution to prevent a carry-over contamination, a 10 µL of 2× Anyplex PCR master mix (Seegene Inc.) including DNA polymerase and buffer with deoxynucleoside triphosphates (dNTPs). The reaction mixture tube was agitated by inverting it five times or by quickly vortexing. A 15 µL of the reaction mixture was dispensed into 0.2-mL polymerase chain reaction (PCR) tubes. The nucleic acid of each sample was added to the reaction mixture tube at a volume of 15 µL in order to reach a total reaction volume of 20 µL. The real-time PCR was performed using a CFX96 real-time PCR system (Bio-Rad, Hercules, CA, USA) in the following conditions: 15 minutes at 95℃, followed by 15 cycles of 15 seconds at 95℃, 30 seconds at 60℃ and then 35 cycles of 30 seconds at 95℃, 32 seconds at 60℃.
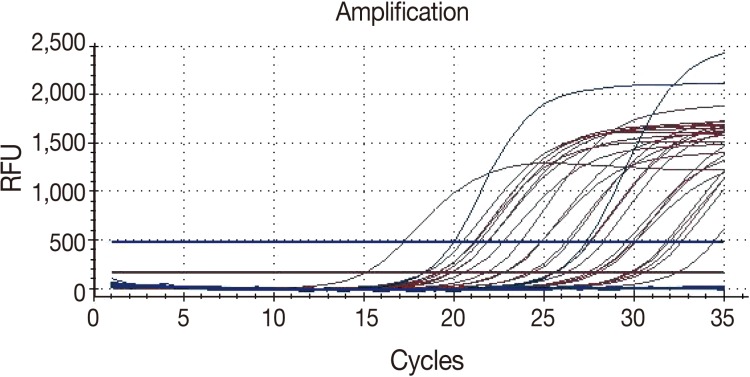
For the real-time PCR, the cycle threshold (Ct) was defined as the cycle at which a significant increase in fluorescence is detected. The specimen and the internal control, whose Ct value was <40, were considered positive controls (Fig. 1). Each run contained both a positive and negative control. In addition, if there were any false-negative or false-positive reactions of the control, all the experimental procedures were repeated again.
Statistical analysis
All statistical analyses were performed with SPSS ver. 17 (SPSS Inc., Chicago, IL, USA). To evaluate the possible relationships between the BRAF mutation and various clinicopathologic parameters, we used either the Fisher's exact test or Mann-Whitney test. Using the Mann-Whitney test, we analyzed the relationship between the BRAF mutation and ordinal clinicopathologic variables including Breslow thickness and tumor, node and metastasis (TNM) stage. In addition, we used the Kaplan-Meier method to analyze the impact of the BRAF mutation on overall survival (OS) and disease-free survival (DFS). Then, we compared these values using a log-rank test. A p-value of <0.05 was considered as statistically significant.
RESULTS
Clinicopathologic characteristics of primary cutaneous and non-cutaneous melanomas
There were 58 patients with primary cutaneous melanoma, who consisted of 24 men and 34 women. At the time of diagnosis, the mean age of patients with primary cutaneous melanoma was 58.3 years (range, 16 to 86 years). In addition, patients with primary cutaneous melanoma were composed of 38 cases of acral lentiginous melanoma (ALM), 16 cases of nodular melanoma (NM), and 4 cases of superficial spreading melanomas (SSM). The mean thickness of the tumor was 5.8 mm (range, 0.5 to 50.0 mm). In patients with primary cutaneous melanoma, there were nine cases (15.5%) of nodal involvement and 13 cases (22.4%) of distant metastasis. The median follow-up period was 12.5 months (range, 1 to 170 months). During the follow-up period, 13.8% (8/58) of patients with primary cutaneous melanoma died of the disease progression. There were 14 cases (24.1%) of recurrence after initial diagnosis (Table 1).
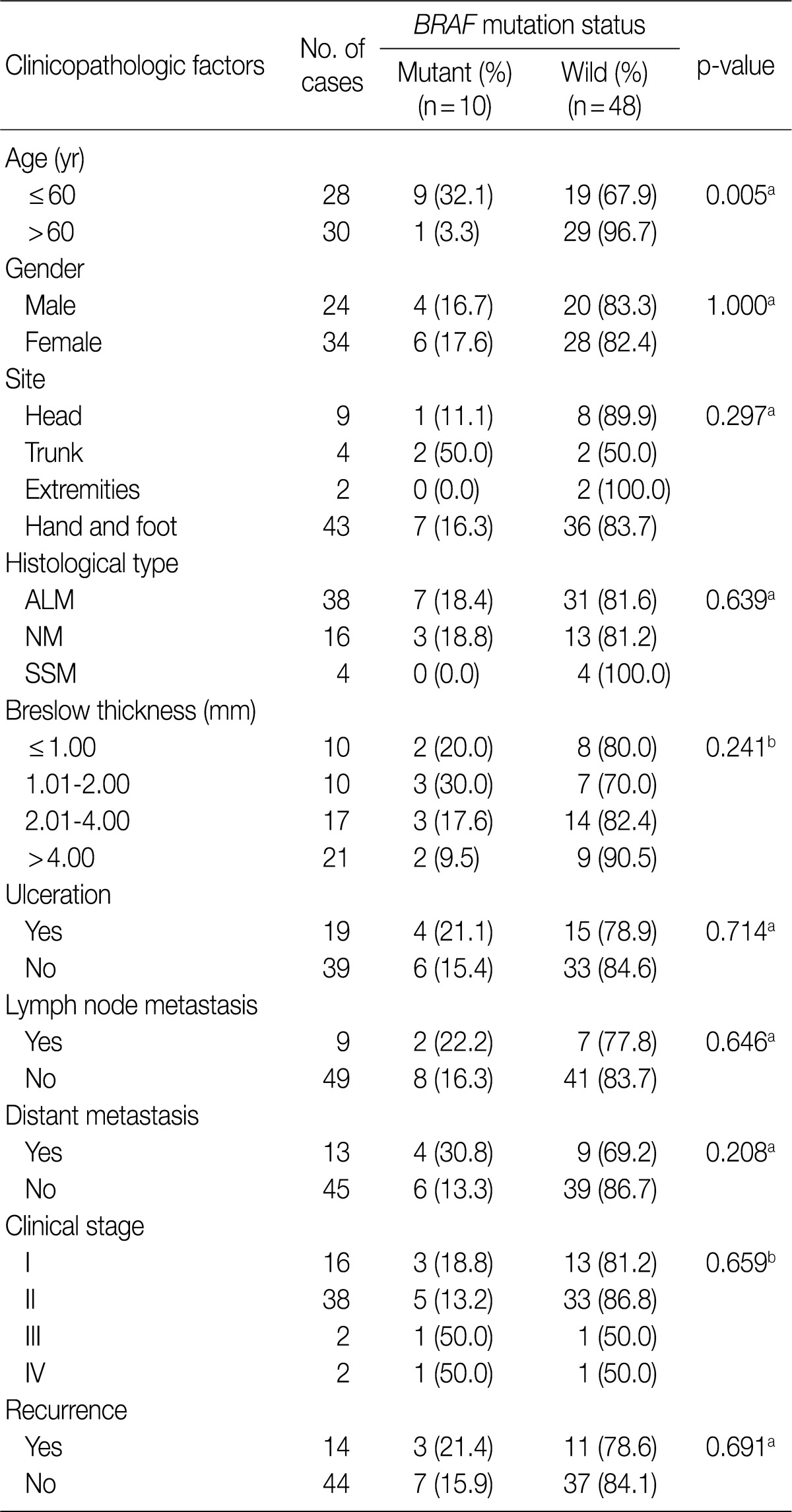
Correlation of the BRAF mutation with various clinicopathologic factors in 58 patients with primary cutaneous melanoma
In the current study, we also enrolled 27 patients with non-cutaneous melanoma who comprised 12 cases of anorectal cancer, five cases of nasal or paranasal cancer, three cases of oral cancer, six cases of ocular cancer, and one case of soft-tissue melanoma. At the time of diagnosis, the mean age of these patients was 60.3 years (range, 42 to 77 years). These patients were composed of 13 men and 14 women. In patients with non-cutaneous melanoma, there were five cases (17.8%) of nodal involvement and nine cases (33.3%) distant metastasis. The median follow up period was 3 months (range, 1 to 125 months). During the follow-up period, 22.2% (6/27) of patients with non-cutaneous melanoma died of the disease progression. There were nine cases (33.3%) of recurrence.
Correlation between the BRAF mutation and clinicopathologic parameters in primary cutaneous and non-cutaneous melanomas
The BRAF mutation was found in 17.2% (10/58) of patients with primary cutaneous melanoma (n=58). We analyzed the correlation between the frequency of the BRAF mutation and other clinicopathologic parameters, whose results are summarized in Table 1.
In patients with primary cutaneous melanoma, the age was the only variable that had a significant correlation with the BRAF mutation. The frequency of the BRAF mutation was significantly higher in patients aged 60 years or younger as compared with those aged 60 years or older (p=0.005). There was no significant difference in the frequency of the BRAF mutation between men and women (p=1.000). In addition, we also analyzed the correlation between the specific site of the occurrence of primary cutaneous melanoma and the frequency of the BRAF mutation. This showed that there was no significant correlation between the two variables (p=0.297).
The BRAF mutation was found in 18.4% (7/38) of patients with ALM and 18.8% (3/16) of patients with NM. In addition, it was not found in four patients with SSM. There were no significant differences in the frequency of BRAF mutation between these histological subtypes (p=0.639).
The mean thickness of the tumors was smaller in patients with the BRAF mutation as compared with those without the BRAF mutation (4.8±7.2 mm vs 6.0±8.4 mm). The frequency of BRAF mutation was higher in patients with a Breslow thickness of ≤2 mm as compared with those with a Breslow thickness of >2 mm (25.0% vs 13.2%). Neither the mean tumor thickness nor the Breslow thickness were variables that had a significant correlation with the frequency of BRAF mutation (p=0.328 and p=0.241, respectively).
We did not find a significant association between the presence of distant metastasis and the BRAF mutation, although the frequency of the BRAF mutation was slightly higher in patients with distant metastasis (30.8%) as compared with those without distant metastasis (13.3%).
We also evaluated if the AJCC clinical stage, presence of ulceration, nodal metastasis and recurrence are significantly correlated with the frequency of the BRAF mutation. But none of these factors had a statistically significant correlation with the frequency of the BRAF mutation. The frequency of BRAF mutation had no statistically significant impact on both the OS and DFS (p=0.561 and p=0.397, respectively). In patients with the BRAF mutation, however, there was a slightly increased tendency in the OS and DFS (Fig. 2).
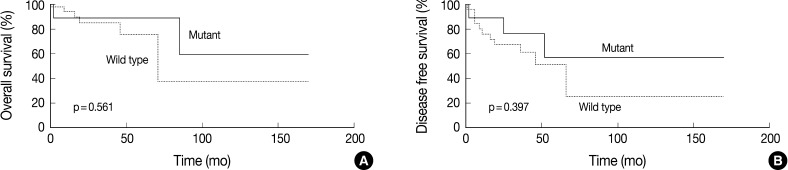
Kaplan-Meier survival curves for overall survival (A) and disease-free survival (B) stratified by the BRAF mutation in primary cutaneous melanoma.
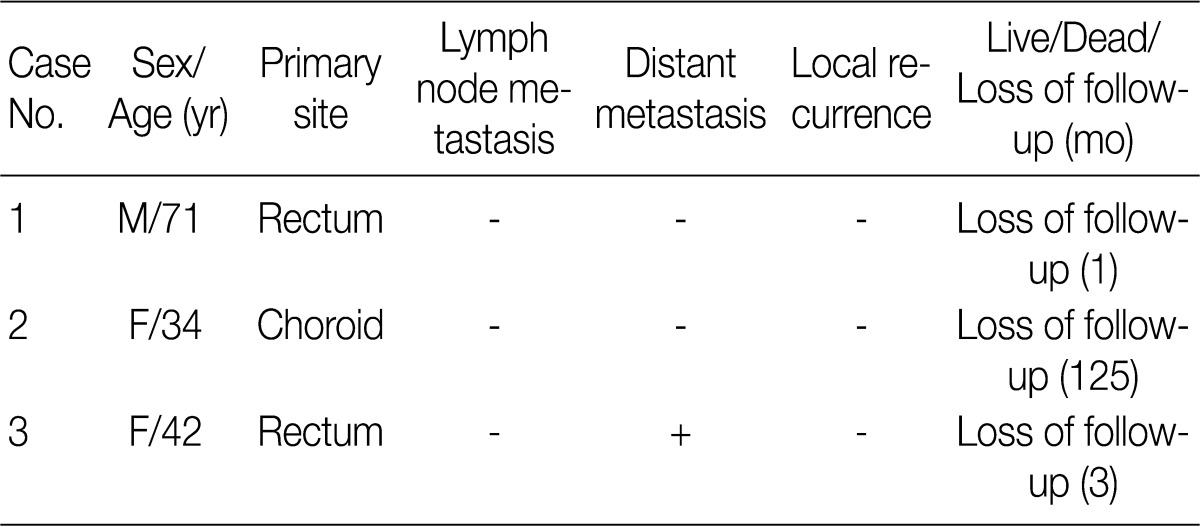
The BRAF mutation was found in 11.1% (3/27) of patients with non-cutaneous melanoma. In these three patients, the clinical characteristics are summarized in Table 2.
DISCUSSION
Our results showed that the frequency of BRAF mutation was 17.2% in 58 patients with primary cutaneous melanoma. This value is relatively lower than previous reports (20-80%),6 most of which employed the microdissection, but, we extracted the genomic DNA from the entire sections without a microdissection technique. This methodological difference, however, is not sufficient to explain the low frequency of the BRAF mutation seen in our results. Yazdi et al.7 compared the frequency of the BRAF mutation between the microdissected samples and those the DNA was extracted from an entire section without using a microdissection technique. There was no significant difference in the frequency of the BRAF mutation between the two groups. Other reasons for the difference in the frequency of BRAF mutation may be that there is a variability in types of BRAF mutation, the proportion of histological subtypes and the sensitivity of the methods for detecting it. Some of the previous studies have detected various types of BRAF mutations including exon 11 mutations (G465A, G465E, and G468S changes) as well as exon 15 mutations (V600E, V600K, V600R, V600G, and V600D changes) although the BRAF V600E mutation accounted for more than 90% of the detected mutations. In addition, of note, because most of the previous studies have been conducted in the Caucasian population, it was unavoidable that they enrolled a higher proportion of patients with SSM or NM than those with ALM. In the current study, however, 65.5% (38/58) of total patients with primary cutaneous melanoma corresponded to cases of ALM. It has also been reported that ALM has a low frequency of BRAF mutation compared to other subtypes of melanomas.6,8 According to a recent study that has been conducted in the Asian population, the frequency of BRAF mutation was 15.0% and this value was similar to our results.9 The above mentioned reasons make it more difficult to interpret our results that the frequency of BRAF mutation was relatively lower as compared with previous published studies.
Our results showed that the age was the only clinicopathologic variable that has a significant correlation with the frequency of the BRAF mutation in patients with primary cutaneous melanoma. Patients aged 60 years or younger had a higher frequency of the BRAF mutation as compared with those aged 60 years or older, which is consistent with previous reports.10-13 In older patients, the melanoma has a predilection for the skin area that is vulnerable to chronic sun damage, such as the face, and it also has a very slow growth. In younger patients, however, the melanoma is more likely to occur on the sun-exposed skin without causing chronic sun damage or in relatively sun-protected sites. In addition, it can also be caused by both genetic and intrinsic factors.14,15 Our results also showed that of the ten patients, only one case of the BRAF mutation occurred on the face of a female patient older than 60 years. In addition, to rule out the effects of histological subtypes, the site of occurrence and the degree of sunlight exposure, we also examined the correlation of the BRAF mutation with the age in patients with ALM. This showed that the frequency of the BRAF mutation was higher in patients younger than 60 years than those older than 60 years in cases of ALM (p=0.011). These results indicate not only that the age is an independent variable that is correlated with the BRAF mutation but also that there is a difference in the pathogenesis between the two groups. This cannot be clarified. It can be speculated, however, that the difference in the pathogenesis between the two age groups may be due to the mutations of NRAS and BRAF, both of which are mutually exclusive events in the pathogenesis of MM.4,16 Previous studies have demonstrated that the NRAS mutation is associated with older age, thicker tumor and poor clinical outcome as compared with the BRAF one.10,17 Based on these reports, we can assume that the BRAF mutation is more commonly involved in the pathogenesis of melanoma in younger patients. We can also presume that the NRAS mutation play a more predominant role in the pathogenesis of melanoma in older patients.
Several previous studies have examined the correlation between the prevalence of the BRAF mutation and histological subtypes. These studies have reported that SSM and NM more frequently harbor BRAF mutations than ALM.6,8 In the current study, as compared with previous published data, the frequency of BRAF mutation (18.4%) seen in patients with ALM was similar but that seen in patients with SSM or NM was relatively lower (0% and 18.8%, respectively). Presumably, this might be because the current study enrolled a relatively smaller number of patients with SSM or NM. Further large-scale studies are therefore warranted to identify the correlation between the frequency of BRAF mutation and histological subtypes in Korean patients with cutaneous melanoma.
Our results showed that the frequency of BRAF mutation had an inverse correlation with tumor thickness despite a lack of the statistical significance. Several previous reports have also shown that the frequency of BRAF mutation is associated with decreased tumor thickness.11,17 These observations indicate that the frequency of BRAF mutation may be related to more differentiated forms of cutaneous melanoma and a slower cell proliferation rate. But there were no other studies that have revealed this correlation.10,18
In our series, the frequency of BRAF mutation had no significant correlations with the clinical stage, metastasis and cumulative survival rate. To date, several studies have also revealed that the frequency of BRAF mutation is not correlated with a prognosis and a survival in patients with primary cutaneous melanoma.18-20 A lack of consistency and correlation may be due to the inclusion of a variety of histological subtypes and advanced tumors, as well as the inclusion or exclusion of NRAS mutant cases, known as an adverse prognostic factor, from the wild type category.10,17 In addition, the study size may not have had sufficient power to detect moderate differences in prognosis and survival. Further large-scale, well-controlled studies are therefore warranted to examine the correlations between clinicopathologic parameters and the status of both the BRAF and NRAS mutation, which would be essential for clarifying the relationship between these mutations and clinical outcomes.
In the current study, the BRAF mutation was found in only 11.1% (3/27) of patients with non-cutaneous melanoma. These three patients comprised one case of uveal melanoma and two cases of rectal melanoma. As described here, a lower prevalence of the BRAF mutation seen in patients with non-cutaneous melanoma is also in agreement with previous reports; the frequency of the BRAF mutation (0-9.5%) was relatively lower in non-cutaneous melanomas as compared with their cutaneous counterparts.6,12,21 Three previous studies could not detect a single BRAF mutation in a total of 83 patients with uveal melanoma. 21-23 But, we detected the BRAF mutation in one case of uveal melanoma. The melanocytes and their progenitor cells can undergo malignant transformation with no respect to the anatomical location. Based on the unequivocal differences in the incidence, the profile of sun-exposure and clinical behavior between two melanomas, however, we can assume that cutaneous and non-cutaneous melanomas may not necessarily share identical pathways of tumorigenesis. Curtin et al.24 elucidated the important role that the KIT plays in the pathogenesis of ALM and mucosal melanomas which harbor the BRAF mutations at lower frequencies. According to these authors, mutations and/or copy number increases of KIT were observed in 39% of mucosal melanomas, 36% of ALM and 28% of melanomas that occurred on the skin which was vulnerable to chronic sun-damage. By contrast, there were no melanomas with KIT aberration that occurred on the skin without chronic sun-damage, predominantly occurring in the Caucasian population. In addition, the functional alteration of the p16INK4a/CCND1/CDK4/Rb pathway might also be associated with the pathogenesis of ALM. Although the role of CCND1 was less well established in melanomas, recent studies have revealed frequent amplifications (~45%) of the CCND1 gene in ALM.25,26 These different genetic alterations indentified in melanomas at different numerous sites and with varying levels of sun exposure indicate that there are distinct genetic pathways in the development of various melanoma subtypes.
Based on the recent identification of several key molecular pathways whose association with the pathogenesis of melanoma might be plausible, new targeted therapies have been developed for the treatment of this devastating disease. BRAF-specific inhibitors, such as RAF-265 (CHIR-265) and PLX-4032 (Plexikkon), and MEK-specific inhibitors, such as PD0325901 (Pfizer Oncology) and ARRY-142886 (AZD6244), are currently being tested in clinical trials.27 Several preclinical and early clinical studies have shown that responses to these agents are associated with the BRAF mutation. Ongoing clinical trials provide hope for an improved progression-free survival and minimization of unexpected toxicities in patients with advanced melanoma. In Korea, however, ALM is a more prevalent type of cutaneous melanoma and it demonstrates a decreased number of BRAF mutations as compared with SSM and NM. To facilitate the development of effective targeted therapies for the treatment of melanomas in the near future, it is imperative that further studies be conducted to examine the prevalence and clincopathologic significance of the alterations of several genes which may contribute to the pathogenesis of melanoma in a Korean population, including KIT, CCND1 and NRAS as well as BRAF gene.
In conclusion, our study provides preliminary results concerning the frequency of the BRAF mutation and its association with clinicopathologic parameters in Korean patients with primary cutaneous or non-cutaneous melanoma. Further large-scale, well-controlled studies are warranted to examine the correlations between clinicopathologic parameters and the alterations of other potential genes. This will enable us to identify more accurate frequency of the alterations of each gene, to improve an understanding of the molecular pathogenesis of melanoma and to develop new diagnostic/prognostic markers and targeted drug therapies.
Acknowledgments
This work was supported by the Dong-A University research fund.
Notes
No potential conflict of interest relevant to this article was reported.