Tumor Budding and Recurrence in Submucosal Invasive Colorectal Cancers of Favorable Histology: Case Reports of Two Early Colorectal Cancers with Advanced Recurrences
Article information
Abstract
Complete resection of submucosal invasive colorectal cancer (SICC) showing favorable histology is regarded as curative. We report on two cases of SICC showing recurrence within 5 years despite complete resection. The first patient was a 68-year-old woman with well differentiated rectal adenocarcinoma invading the superficial submucosa, which recurred after 4.7 years. The second patient was a 53-year-old man with pT1N0 moderately differentiated colonic adenocarcinoma. He developed widespread tumor recurrence after 3.9 years. Retrospective pathologic review of the original tumors showed multiple foci of tumor budding at the invasive front. Immunohistochemical staining for D2-40 of deeper levels of the paraffin blocks showed rare foci of small lymphatic invasion. Tumor budding at the invasive front may be an important indicator for SICC aggressiveness or may reflect early lymphatic invasion. More aggressive pathologic examination and follow-up is required for patients with SICC showing tumor budding, even in the absence of unfavorable histologic findings.
Recent advances in endoscopic instrumentation and techniques have led to a marked increase in the detection and endoscopic resection of early colorectal carcinomas.1 Although endoscopic complete resection of early colorectal cancer is currently accepted as curative therapy due to the relatively low risk of lymph node metastasis,2,3 about 10% of patients with submucosal invasive colorectal carcinoma (SICC) have synchronous metastases to regional lymph nodes.2,4 It is therefore necessary to stratify patients with SICC into low-risk and high-risk groups to determine the risks of lymph node metastasis5 and to elect proper additional management. The pathological features associated with lymph node metastasis in patients with SICC include poor differentiation, deep invasion into the submucosal layer, and lymphatic channel invasion.3,6,7 More recently, tumor budding has been shown to reflect the biologic aggressiveness of colorectal cancers. Herein, we describe two patients with small SICC and tumor budding, resulting in adverse outcomes within 5 years, despite favorable tumor histology and complete resection.
CASE REPORTS
Case 1 was a 68-year-old female patient who was admitted for further treatment of an incidentally detected rectal polyp. Colonoscopic examination (Fig. 1A) showed a 2 cm-sized semipedunculated polyp, 7 cm from the anal verge. A biopsy showed an adenocarcinoma with a background of tubulovillous adenoma. Since preoperative computed tomography (CT) showed no evidence of lymph node or distant metastasis, she underwent transanal endoscopic microsurgery with full thickness of excision in August 2003. Histologic examination revealed a 3.5 mm-sized, well differentiated adenocarcinoma invading the submucosa at a depth of 1.2 mm from the muscularis mucosa (sm1; thickness of submucosal layer 4 mm) (Fig. 1B). Since no lymph nodes were included within the perirectal fat, the pathologic stage was pT1NX. The nearest safety margin to carcinoma was 1.3 cm (deep margin). There was no definitive evidence of lymphatic invasion at first pathologic diagnosis, and she therefore received no additional therapy. In April 2008, an abdominal CT performed during routine follow-up revealed a 2 cm-sized nodular lesion in her perirectal fat (Fig. 2A). Anterior resection was performed, and histologic examination showed that this tumor, which involved the perirectal adipose tissue with no metastasis out of 15 regional lymph nodes (rpT0N1c), was compatible with a recurrence of the previous rectal cancer (Fig. 2B).

Endoscopic and histologic findings of primary tumor in case 1. (A) Colonoscopic examination shows subpedunculated mass in the rectum with an ill-defined boundary. (B) Pathologic examination reveals a rectal cancer infiltrating the submucosa, with an adenoma component at the periphery.
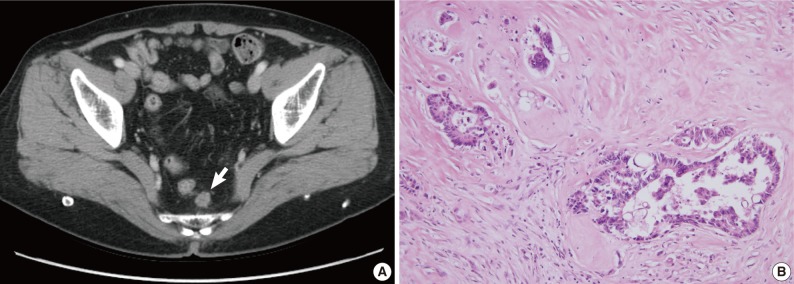
Radiologic and histologic findings of recurrent tumor in case 1. (A) A newly appeared soft tissue mass (arrow) in the perirectal tissue is detected upon computed tomographic imaging. (B) A perirectal mass shows an adenocarcinoma with intraluminal necrosis.
Case 2 was a 53-year-old male patient with tenesmus that had undergone a colonoscopy, which revealed a 1 cm-sized flat-elevated lesion with central depression in the sigmoid colon, 25 cm from the anal verge (Fig. 3A). He underwent anterior resection in March 2004, and histologic examination showed that the tumor was a 5 mm-sized, moderately differentiated adenocarcinoma invading the submucosa (sm3) (Fig. 3B). The depth of submucosal invasion was 2.5 mm and the safety margin was at least 3 cm. No metastases were detected out of six pericolic lymph nodes, and the patient did not receive any adjuvant treatment. In February 2008, the patient developed widespread recurrence of the cancer (Fig. 4A) and underwent a low anterior resection with paraaortic lymph node dissection and segmental resection of the small intestine. Pathologic examination revealed a recurrent adenocarcinoma extended to the periureteral soft tissue and multiple metastases to the small intestine, regional and para-aortic lymph nodes (rpT4N2bM1b) (Fig. 4B).
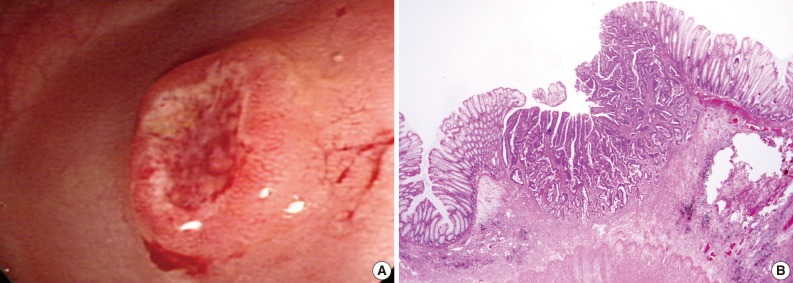
Endoscopic and histologic findings of primary tumor in case 2. (A) Colonoscopic examination shows an elevated tumor with central depression in the sigmoid colon. (B) Pathologic examination reveals a colon cancer invading the submucosa.
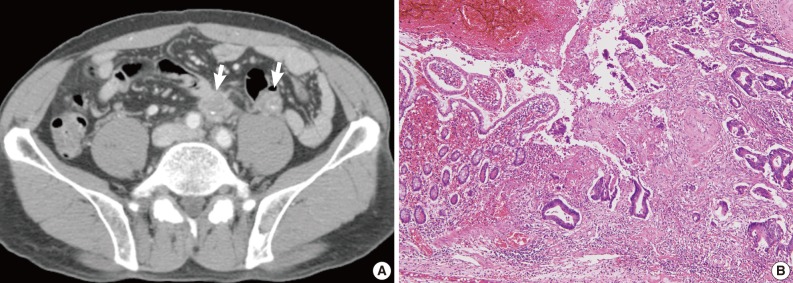
Radiologic and histologic findings of recurrent tumor in case 2. (A) Multiple metastatic lesions (arrows) in the para-aortic lymph node and mesentery are identified on computed tomographic imaging. (B) Adenocarcinoma involves the small intestinal mucosa, with similar histological findings to the original tumor (Fig. 2B).
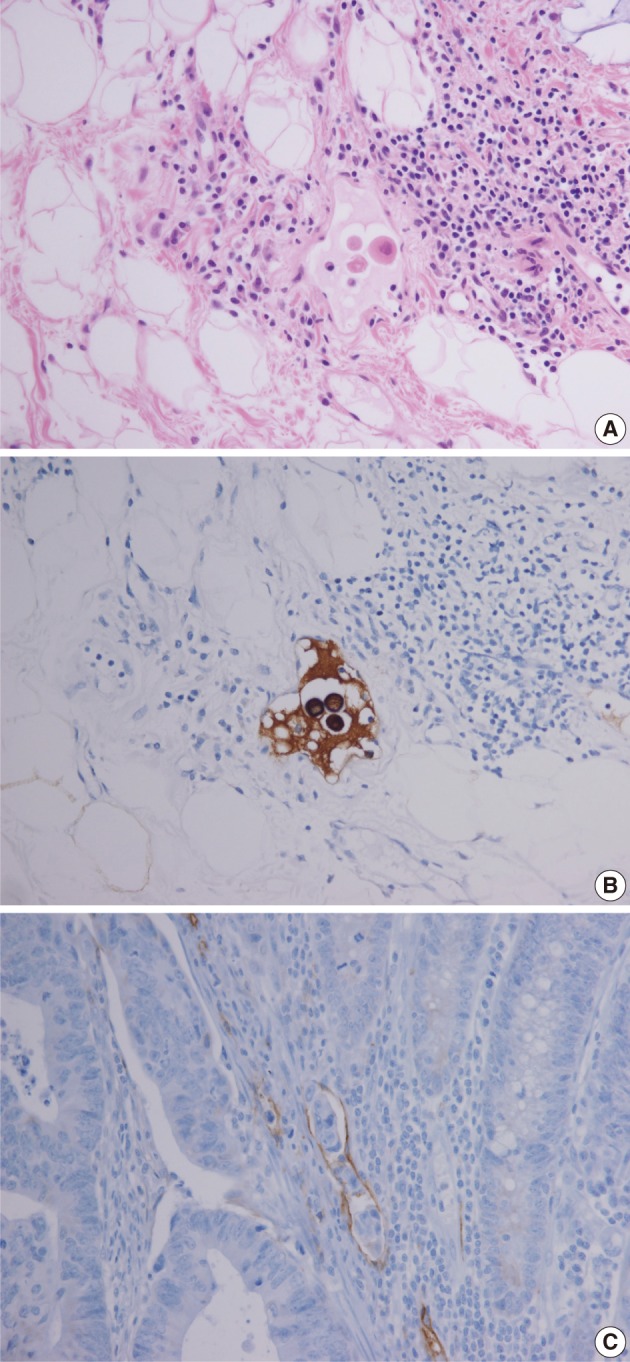
Retrospective pathologic review of the two primary carcinomas showed tumor buddings, defined as multiple foci of a single cancer cell and/or a micro-nest composed of several tumor cells, at the invasive front of both primary tumors (Fig. 5). A magnification of ×400 showed more than 10 foci in both primary tumors. In addition, small endolymphatic tumor emboli were detected only after deeper cutting of the paraffin blocks in both cases. These minute lymphatic invasion were not observed on the orginial hematoxylin and eosin slides. In both patients, the presence of tumor budding and lymphatic invasion was confirmed by immunohistochemical assays for cytokeratin and D2-40, respectively (Fig. 6). Immunohistochemical staining was performed using a mouse monoclonal antibody for cytokeratin (1:500, Dako, Glostrup, Denmark) and D2-40 (1:200, Dako).
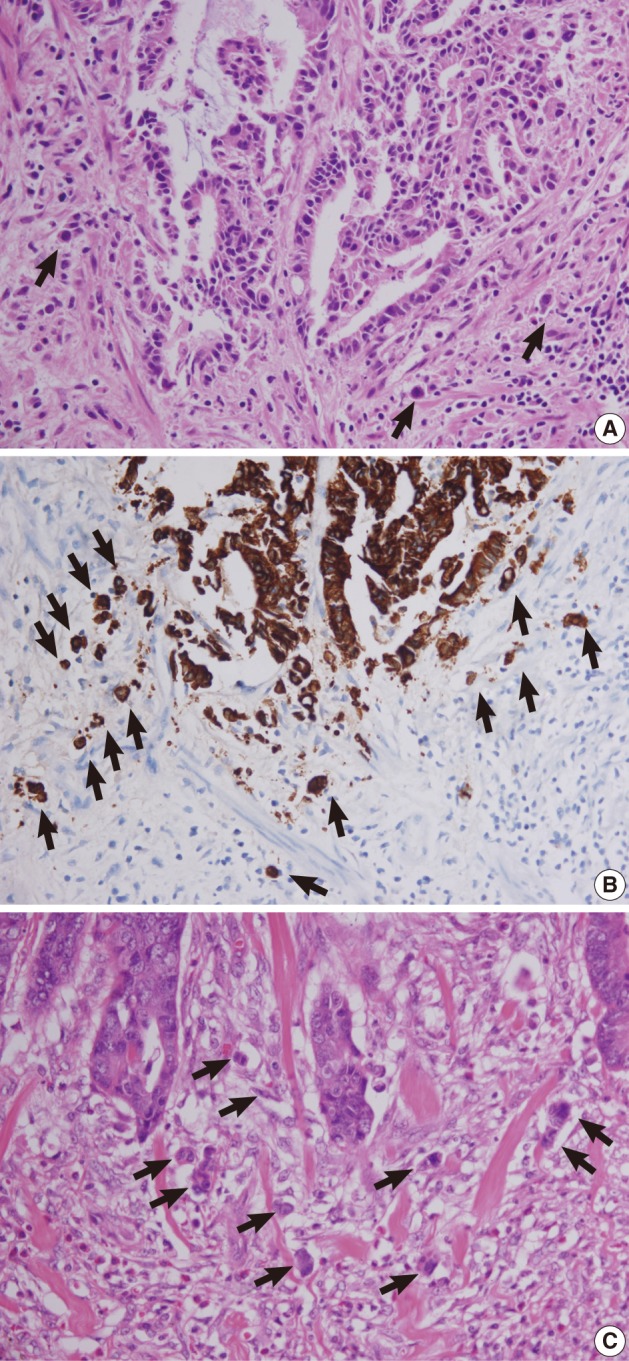
Tumor buds at the invasive front of primary tumors (arrows). (A, B) In case 1, subtle budding cells (A) around the tumor margins are highlighted by immunohistochemistry using antibody to cytokeratin (B). (C) In case 2, frequent tumor budding is observed in a high power field.
DISCUSSION
Complete endoscopic resection of intramucosal carcinomas in the colorectum is regarded as curative, as there is no risk of lymph node metastasis.2,8 In contrast, lymph node metastasis is present in 6-12% of patients with SICC. Positive lymph node status has been reported to be the only significant independent predictor of 5-year cancer specific survival and 5-year disease-free survival in patients with pT1 and pT2 colorectal cancers.9 The standard of care for node-positive colorectal cancer patients is adjuvant chemotherapy based on 5-flourouracil regimens.10 Therefore, it is critical to evaluate the likelihood of lymph node metastasis in SICC. Poorly differentiated histology, presence of deep submucosal invasion, and lymphatic invasion are predictors of lymph node metastasis in patients with T1 colorectal cancer.3,6,7 Most T1 lesions without adverse pathologic features can be locally excised because the risks of local recurrence and lymph node metastasis are very low. However, a T1 lesion with unfavorable histologic features has a high risk of local recurrence and lymph node metastasis, whether metachronous or synchronous, indicating the need for additional treatment,2 since the prognosis is worse for patients who undergo additional surgery after local recurrence or lymph node metastasis.
Tumor budding, or the presence of isolated single tumor cells or small cell clusters (up to 4 cells) scattered in the stroma at the invasive tumor margins, has been considered a malignant characteristic of colorectal carcinomas.11,12 Tumor budding is observed in many colorectal carcinomas and has been reported to be a useful predictor of patient outcome13,14 and lymph node metastasis6,7,12,13,15 in T1 colorectal cancer. Rectal cancer patients having a tumor budding intensity of >10 foci within a microscopic field of ×250 had lower 5-year survival rates than patients with tumor budding intensity of <10. Moreover, high-grade "tumor budding activity," defined as 10 or more foci in a microscopic field of ×200, has been closely associated with lymphatic involvement and lymph node metastasis in SICC.16 Immunophenotypically, loss of E-cadherin in budding cells of colorectal cancers has been reported, with loss of E-cadherin within the main tumor body being significantly associated with tumor budding.17
Both patients described here had tumors with moderate to well differentiated histology and safety margins were sufficient for complete resection. However, recurrent disease developed within relatively short intervals. In the first patient, tumor recurrence without lymph node metastasis was observed 4.7 years after local tumor excision. In the second patient, both locoregional and systemic recurrences were observed 3.9 years after surgical resection, although lymph node metastasis was not identified in the primary tumor. Since a study of endoscopically-resected SICC demonstrated that recurrent primary cancers were significantly associated with poorly differentiated histology,18 we believed that other factors may have contributed to disease recurrence in these 2 patients. A thorough retrospective histologic review revealed frequent tumor budding from both tumors. Lymphatic channel permeation was not initially apparent on hematoxylin and eosin staining alone but was detected only after deeper cutting of the paraffin blocks and additional immunohistochemical assays. Similar to our patients was a patient with a submucosal invasive colon cancer of the pedunculated type, who developed lung metastasis 6 years after curative endoscopic polypectomy.19 The lesion was a 1 cm-sized, well differentiated adenocarcinoma showing tumor budding in the invasive front without lymphatic invasion, suggesting that tumor budding may be a significant prognostic factor for cancer recurrence and distant metastasis. Moreover, tumor budding may be closely associated with lymphatic channel involvement and lymph node metastasis. Although lymphatic invasion is regarded as an important prognostic factor for patients with SICC, lymphatic invasion may be subjective and be overestimated or underestimated due to retraction artifacts related to tissue processing for paraffin blocks.20 In contrast to angiolymphatic invasion, detection of tumor budding is more objective. Moreover, tumor budding may reflect early lymphatic invasion, even in the absence of definite lymphatic invasion. Consequently, meticulous pathologic examination is needed to detect tumor budding and angiolymphatic invasion in patients with SICC. Furthermore, immunohistochemical staining for D2-40 and cytokeratin may be helpful in detecting tumor budding and lymphatic channel permeation.
To our knowledge, this is the first report of cases in Korea of SICC showing tumor budding with advanced recurrences. Multiple tumor budding at the invasive front may predict tumor aggressiveness in SICC. Further pathologic examination for lymphatic invasion such as deeper section or immunohistochemisty is needed in cases with SICC that have frequent tumor budding in which lymphatic invasion is not seen in the original hematoxylin and eosin slides. Besides, more aggressive follow-up is necessary for SICC patients showing tumor budding, even in the absence of unfavorable histologic findings. Other supplementary treatment measures, such as adjuvant chemotherapy, may be required to prevent recurrence and metastasis in these patients.
Notes
No potential conflict of interest relevant to this article was reported.