Cytologic Findings of Thyroid Carcinoma Showing Thymus-like Differentiation: A Case Report
Article information
Abstract
Carcinoma showing thymus-like differentiation (CASTLE) is a rare carcinoma of the thyroid or adjacent soft tissue of the neck with a histologic resemblance to thymic epithelial tumors. Although the fine-needle aspiration (FNA) plays a central role in the initial evaluation of thyroid nodules, few reports about the cytologic findings of CASTLE have been found according to a review of literatures. We report cytologic findings of a case of CASTLE. A 34-year-old woman presented with a 2-month history of sore throat. The FNA showed that the smear was composed of three dimensional clusters and sheets. The tumor cells were round to ovoid with high nuclear : cytoplasmic ratios. The nuclei were vesicular with small nucleoli. There were some tumor cells showing keratinization. Some lymphocytes were found on the background and within clusters. The presence of poorly-differentiated tumor cells with a focal keratinization and a lymphocytic background on the FNA is suggestive of CASTLE.
Carcinoma showing thymus-like differentiation (CASTLE) is a rare carcinoma of the thyroid gland or adjacent soft tissue of the neck with a histological resemblance to thymic epithelial tumors.1 Its incidence in thyroid malignant tumor is estimated at 0.075%.2 Although the fine-needle aspiration (FNA) biopsy plays a central role in the initial evaluation of solitary thyroid nodules, few reports about the cytologic findings of CASTLE have been found according to a review of literatures.3-6 In Korea, three cases of CASTLE have been reported in the literature but its cytologic findings have not been described.7-9 We report cytological findings of CASTLE in a 34-year-old woman.
CASE REPORT
A 34-year-old woman presented with a 2-month history of sore throat. The patient had no notable medical or family history. Physical examination revealed a palpable right thyroid mass. Thyroid function and calcium tests were within normal limits. Ultrasound showed a 1.7-cm, well demarcated, heterogeneous, low echoic solid mass in the lower pole of the right thyroid (Fig. 1). A computed tomography scan of the neck revealed a 3.3-cm, exophytic, low-attenuated mass in the lower pole of the right thyroid. The FNA biopsy of the mass was performed, which was accompanied by the standard Papanicolaou stain.
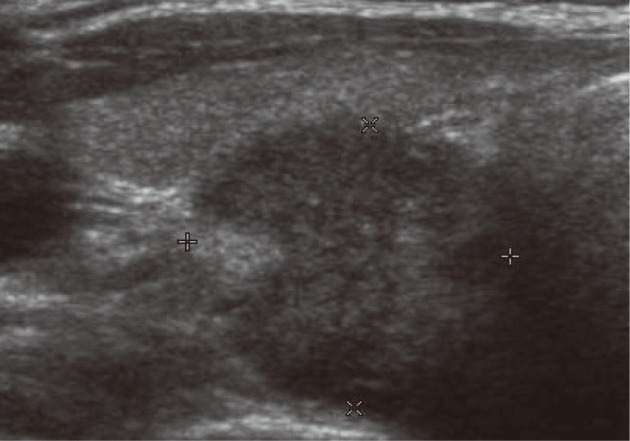
Thyroid ultrasonography shows a 1.7-cm, well-defined, hypoechoic, solid mass in the lower pole of the right thyroid.
The smear was composed of not only cohesive three dimensional clusters and sheets but also singly scattered cells (Fig. 2A). The tumor cells showed mild anisonucleosis and high nuclear : cytoplasmic (N/C) ratios. Nuclei were round to ovoid with irregular nuclear contours and small, distinct nucleoli (Fig. 2B). The nuclear chromatin was vesicular or coarsely granular. Nuclear grooves were rarely seen. Cytoplasm was scanty and amphophilic. Cytoplasmic border was indistinct. Some tumor cells showed dense orangeophilic cytoplasm, which is suggestive of individual cell keratinization (Fig. 2C). Some lymphocytes were found on the background and within clusters, which was accompanied by the presence of a few stripped nuclei (Fig. 2D). Mitotic figures were rarely seen. Intranuclear cytoplasmic inclusions were not observed. Papillary or follicular structure was not observed. The aspirate was interpreted as a high-grade malignant thyroid neoplasm without further definitive classification. The total thyroidectomy was performed.
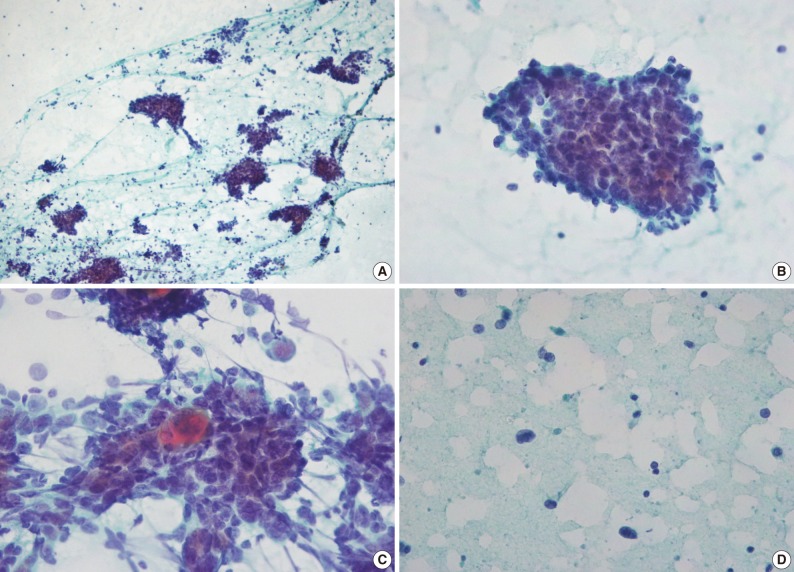
(A) The smear shows cohesive sheets and clusters, and singly scattered cells. (B) Tumor cells have round to ovoid vesicular nuclei, small prominent nucleoli and scanty cytoplasm. A few lymphoid cells are present on the background and within the clusters. (C) Keratinization is seen. (D) Stripped nuclei are present in the background (A-D, Papanicolaou stain).
On gross examination, the thyroid contained a 4.0-cm solid mass replacing most of the right thyroid. The mass had a lobular, tan-colored cut surface and a firm consistency (Fig. 3A). On microscopic examination, the tumor was well-circumscribed and it was composed of variably sized and irregularly shaped lobules of cohesive polygonal tumor cells which were separated by bands of dense fibrous stroma (Fig. 3B). The tumor cells had high N/C ratios, eosinophilic cytoplasm and ill-defined cell borders. The nuclei were vesicular or coarsely granular with prominent nucleoli. The mitotic figures were sparse. There was mild lymphoplasmacytic infiltration within the tumor nodules and septae. Cytoplasmic keratinization was seen. The tumor cells showed positivity for CD5, carcinoembryonic antigen, high molecular weight keratin (HMWK), cytokeratin 5 (CK5), and p63. The tumor was negative for thyroid transcription factor-1, thyroglobulin, and calcitonin. Vascular invasion and extensive extra-thyroidal extension were present. One regional lymph node was found and involved by tumor.
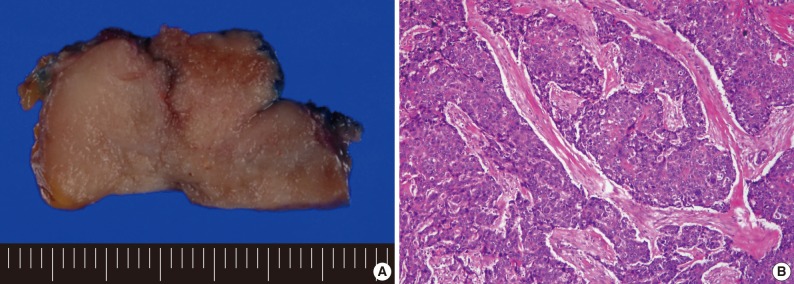
(A) The cut surface of the tumor is lobulated, solid, and tan-colored. (B) Broad anastomosing islands of tumor cells are separated by desmoplastic stroma. Squamous differentiation is present.
Over a 27-month follow-up period, the patient had no evidence of tumor recurrence.
DISCUSSION
The cytological diagnoses were mentioned in previous 14 reports consisting of 40 cases. Malignant tumor or poorly-differentiated carcinoma were the most frequent cytological diagnosis in cases of CASTLE, except for six cases (Table 1).2-15 One case was diagnosed as CASTLE for which no cytological findings have been described.11
In previously reported cases of CASTLE, the most common cytological findings include tight clusters and sheets of round tumor cells with high N/C ratios, vesicular nuclei, prominent nucleoli, amphophilic cytoplasm, and lymphocytic background, which correspond well with the present case.2-4,6 In the present case and those of Hirokawa et al.,2 keratinized cells were present. Youens et al.6 reported indistinct cell borders, intranuclear grooves and sparse mitotic figures, which were also seen in the present case. Hirokawa et al.2 reported intracytoplasmic space surrounded by cell membrane and spindle tumor cells. Youens et al.6 reported not only intranuclear cytoplasmic inclusions and papillary-like structures but also granular chromatin and rosette-like structures. But these findings were not detected in the present case.
The differential diagnoses of CASTLE include papillary carcinoma, Hürthle cell neoplasm, undifferentiated carcinoma, medullary carcinoma and metastatic lymphoepithelioma-like carcinoma.
CASTLE may show intranuclear pseudoinclusions or papillary-like structures, but it does not show fine pale chromatin and monolayer sheets of cells with dense cytoplasm. Intranuclear pseudoinclusions can be seen in various malignant or benign thyroid nodules. Intranuclear pseudoinclusions should be interpreted in light of the other architectural and nuclear features.
Although Hürthle cell neoplasm contains cellular aggregates with abundant cytoplasm and prominent nucleoli, lymphocytic background of CASTLE is lacking. Unlike Hürthle cell neoplasm, CASTLE does not have granular cytoplasm.
It is very difficult to distinguish CASTLE from metastatic lymphoepithelioma-like carcinoma, which also shows sheets of poorly differentiated round cells intermixed with small lymphocytes. Subtle differences, such as frequent mitotic activity and a polymorphous inflammatory background, favor metastatic lymphoepithelioma-like carcinoma.4
CASTLE may resemble undifferentiated thyroid carcinoma with squamous differentiation, but it does not show marked cytologic atypia, frequent mitotic activity and necrotic background characteristic of undifferentiated carcinoma.
Although CASTLE may show rosette-like structures and granular chromatin, along with an abundance of single tumor cells as in medullary carcinoma, it is lacking of typical plasmacytoid cells, pink cytoplasmic granules and amyloid deposition.6
Immunocytochemical study for CD5 is helpful to differentiate CASTLE from other malignant thyroid neoplasm. CD5 is a surface glycoprotein expressed on mature T cells and a subset of B cells.16 Thymic carcinoma cells are also known to be positive for CD5.17 CD5 is almost always expressed in CASTLE. CASTLE is positive for HMWK, CK5, and p63 because it is also a squamous cell carcinoma. However, thyroid squamous cell carcinoma and poorly-differentiated carcinoma were negative for CD5.17 Ito et al.11 reported that the sensitivity and specificity for CD5 immunohistochemistry were 82% and 100%, respectively, in making a diagnosis of CASTLE.
In conclusion, the presence of poorly-differentiated tumor cells with a focal keratinization and a lymphocytic background on the FNA biopsy is suggestive of CASTLE.
Acknowledgments
This work was supported by grant from Inje University, 2008.
Notes
No potential conflict of interest relevant to this article was reported.
