Loss of E-cadherin and Acquisition of Vimentin in Epithelial-Mesenchymal Transition are Noble Indicators of Uterine Cervix Cancer Progression
Article information
Abstract
Background
Epithelial-mesenchymal transition (EMT) has been known to play a key role in the stromal invasion of carcinoma in situ (CIS) lesion. Loss of E-cadherin and acquisition of vimentin are two critical steps in EMT, that are induced by Snail-1 upregulation associated with overexpression of epidermal growth factor receptor (EGFR). However, roles of EMT-related proteins in human cervical tissues have not been fully elucidated. In this study, we investigated the immunoexpressions of EMT-related proteins in CIS, microinvasive squamous cell carcinoma (SCC), and invasive SCC to demonstrate their key roles in tumor progression.
Methods
Eighty one CIS, 17 microinvasive, and 21 invasive SCC cases were immunostained with primary antibodies for Snail-1, EGFR, E-cadherin, and vimentin on paraffin-embedded tissue microarray blocks.
Results
EGFR and Snail-1 proteins were highly expressed but the levels were not significantly different between the three groups. However, loss of E-cadherin and acquisition of vimentin were proven to occur significantly higher in microinvasive and invasive SCC cases than in CIS.
Conclusions
E-cadherin and vimentin were found to be two useful indicators of EMT in evaluating stromal invasion of CIS. However, it was not demonstrated for Snail-1 and EGFR proteins to play any key role in the progression of cervix cancer.
The progression of uterine cervix carcinoma in situ (CIS) to invasive squamous cell carcinoma (SCC) has been known to be associated with epithelial-mesenchymal transition (EMT). EMT is thought to be triggered by diverse growth factors secreted by the tumor and host cells. Epidermal growth factor (EGF) is one of the most representative growth factor that is upregulated or stimulated in the stromal invasion or nodal metastasis. Snail-1 is a zinc-finger transcription factor, which is upregulated by epidermal growth factor receptor (EGFR) signaling and mediates EMT by inducing downregulation of E-cadherin and upregulation of vimentin. To date, there has been only one report on the role of EMT in uterine cervix cancer progression. Their study investigated EGFR overexpression, Snail-1 upregulation, and expression patterns of EMT-related proteins such as vimentin and E-cadherin in human tissues and cell lines.1 Loss or dysfunction of E-cadherin has been known to be associated with the acquisition of invasive capacity and also to be correlated with high tumor grade and a poor prognosis.2,3 EGF and EGFR have been reported as the potent stimulators of cervical cancer cell invasion, which includes lymph node metastasis and recurrence of cervical cancer.1 In an in vitro study, Snail-1 protein was found to be upregulated by EGF stimulation and accumulated in the nuclei of cervical cancer cells.1 The overexpression of Snail-1 protein has been known to be associated with downregulation of E-cadherin and upregulation of vimentin.1,4,5
However, the present study investigated the application of EMT-related proteins as indicators of stromal invasion in the CIS of uterine cervix and their association with the EGFR signaling pathway. This investigation applied the immunohistochemical technique with EGFR, Snail-1, E-cadherin, and vimentin proteins in human uterine cervix tissues including CIS, microinvasive SCC, and invasive SCC cases.
MATERIALS AND METHODS
Patient characteristics
Tissue specimens were obtained from 119 patients with cervical carcinoma who had undergone hysterectomy from 1999 to 2009 at Dankook University Hospital. The current study was approved by the Institutional Review Board (IRB) of Dankook University. Patients' age ranged from 31 to 88 years. They had a median age of 50 and 55 years at diagnosis of CIS and invasive SCC, respectively. They have not received irradiation and anticancer chemotherapy prior to surgery. Stages by the International Federation of Gynecology and Obstetrics (FIGO) classification system (2008) included 81 cases of 0 (CIS), 17 Ia (microinvasive), and 21 Ib-IIIb (invasive). Clinical follow-up periods of microinvasive and invasive groups ranged from 1 to 10 years, with each median period of 5.25 and 2 years. During the follow-up periods, 13 cases of the invasive group (62%) were treated with radiation only or concurrent chemoradiation, but only one of microinvasive group (6%) received an additional radiotherapy. Neither recurrence nor distant metastasis was found in microinvasive carcinoma group, while 5 (24%) out of 21 invasive carcinoma cases showed either recurrent cervix cancer or distant metastasis in lung, liver, kidney, and brain.
Tissue sample preparation by tissue microarray (TMA) technique
Among 119 cases with cervical carcinoma, 59 tumor specimens were paired with their own normal samples for TMA assay to evaluate the staining patterns of E-cadherin, vimentin, EGFR, and Snail-1. In cases with multiple paraffin blocks, the tumor block was matched with a normal one. In an individual case with no more than one available block, each separate area of the tumor and the normal was sampled within the same block. TMA blocks were constructed using pairs of the most representative tumor and normal tissue cores with a diameter of 2 mm, which were obtained from appropriate areas in formalin-fixed paraffin-embedded tissue blocks. These tissue cores were transferred and embedded into the recipient block that had 60 empty 2 mm-sized holes. Using a microtome, 4 µm thick serial sections were cut from the TMA blocks and were transferred to poly-L-lysine-coated slides.
Immunohistochemical staining and its semiquantitative analysis
The microarrayed tissue sections were deparaffinized with standard xylene, rehydrated using graded alcohols, and rinsed with water. The sections were microwaved in 10 mM citrated buffer at 90℃ for 10 minutes and then treated with 3% H2O2-phosphate buffered saline solution to reduce an endogenous peroxidase activity. They were incubated with normal bovine serum to reduce non-specific antibody binding and subsequently subjected to primary antibody reactions. Monoclonal or polyclonal antibodies against EGFR (clone31G7, Invitrogen, Carlsbad, CA, USA), Snail-1 (ab82846, Abcam, Cambridge, MA, USA), E-cadherin (HECD-1, Takara, Shiga, Japan), and vimentin (V9, BioGenex, Fremont, CA, USA) were reacted with the sections for 1 hour at room temperature using dilution ratio of 1:200, 1:800, 1:200, and 1:3,200, respectively. A negative control was incubated without the primary antibodies. Positive controls for E-cadherin and vimentin were matched samples of normal exocervical squamous epithelium and the stromal cells beneath it, respectively. Detection of the immunoreactive staining was carried out by following the avidin-biotin-peroxidase complex method and using the LSAB kit (Dako, Glostrup, Denmark). The sections were subjected to a color reaction with 3,3-diaminobenzidine tetrahydrochloride that contained 3% H2O2 in Tris buffer. They were lightly counterstained with Meyer's hematoxylin.
The immunoexpressions for EGFR, Snail-1, E-cadherin, and vimentin were analyzed as follows: EGFR was interpreted as an overexpression when the tumor cells were immunostained distinctively either as a membranous or cytoplasmic pattern at the area greater than 10% or 50%, respectively.6,7 Upregulation of Snail-1 protein was proven by greater than 50% nuclear immunoreactivity seen at the tumor cells, because its overall immunoreactivity was found to be high with an average of about 50% even in the normal control tissue. Vimentin expression was interpreted as being acquired by the tumor cells, when they exhibited greater than 5% positive immunoreactivity in the tumor. Compared to the EGFR, Snail-1, and vimentin, the E-cadherin with either membranous or cytoplasmic immunoexpression was evaluated qualitatively rather than semiquantitatively as three patterns as follows: 1) strong (S) pattern: E-cadherin staining pattern in almost all tumor cells (>95%) is as strong as in the normal epithelial cells; 2) weak and homogeneous (W&H) pattern: All tumor cells are uniformly stained but more weakly expressed than in the normal squamous epithelium; 3) heterogeneous (HEG) pattern: The intensity of E-cadherin staining differs from cell to cell and the cells without immunostaining are included. W&H and HEG patterns are considered to show either a loss of or reduced E-cadherin expression.8
Statistical analysis
The comparison of each immunoreactivity for EGFR, Snail-1, E-cadherin, and vimentin between three uterine cervix tumor groups and their inter-relationships were analyzed by a Fisher's exact test using SPSS ver. 15.0 (SPSS Inc., Chicago, IL, USA). All tests were two-sided and p-values less than 0.05 were considered statistically significant.
RESULTS
Immunohistochemical staining patterns for four EMT-related proteins in normal and neoplastic squamous epithelial cells
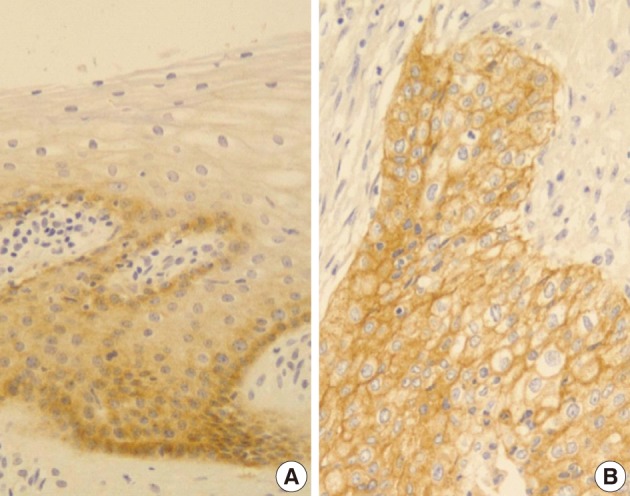
EGFR
Normal cervical mucosal cells expressed much less amounts of EGFR compared to the matched cervical carcinoma cells, mostly showing both membranous and cytoplasmic stainability that are mainly confined to the parabasal and basal layers of the cervix epithelium (Fig. 1A). Cervical carcinoma cells with EGFR overexpression showed diffuse immunoreactivity with honeycomb-like membranous and vaguely cytoplasmic patterns (Fig. 1B).
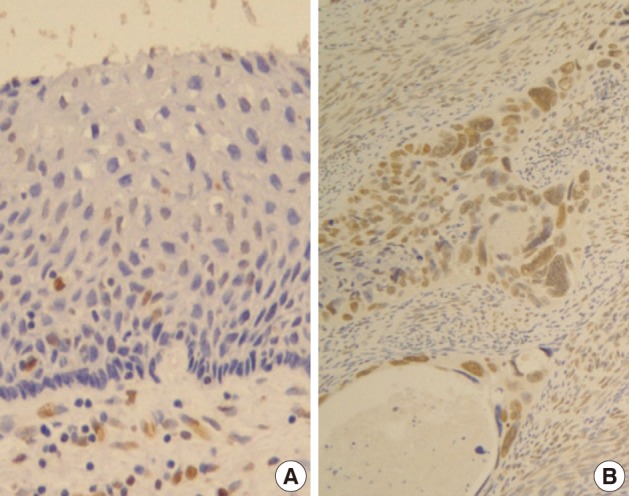
Snail-1
Normal squamous epithelium showed no constant nuclear immunoreactivity (Fig. 2A), whereas invasive squamous carcinoma cells were stained in their nuclei much more than the normal by Snail-1 antibody (Fig. 2B).
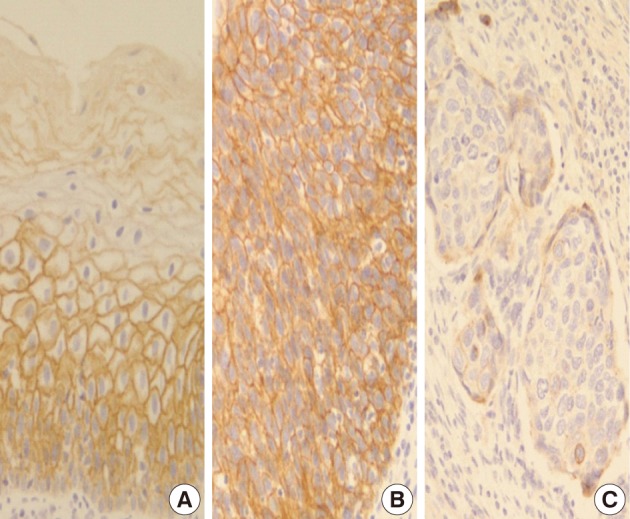
E-cadherin
In normal squamous epithelium, the staining had a fine granular appearance and was limited to the cell surface membrane. The basal layer presented membranous staining with occasional cytoplasmic staining. In the parabasal and intermediate cell layers, E-cadherin immunoreactivity was found circumferentially at the cell membrane, but the staining was lost at the superficial layer (Fig. 3A). This gradually decreasing pattern of membranous staining in the normal cervix was not found in the CIS and invasive SCC cases, which showed weak but diffuse cytoplasmic or heterogeneously reduced immunoreactivity (Fig. 3C) in addition to the strong membranous staining (Fig. 3B). The staining pattern of either reduced or strong membranous immunoreactivity could be found in both CIS and microinvasive carcinoma groups whether the foci was invasive or not.
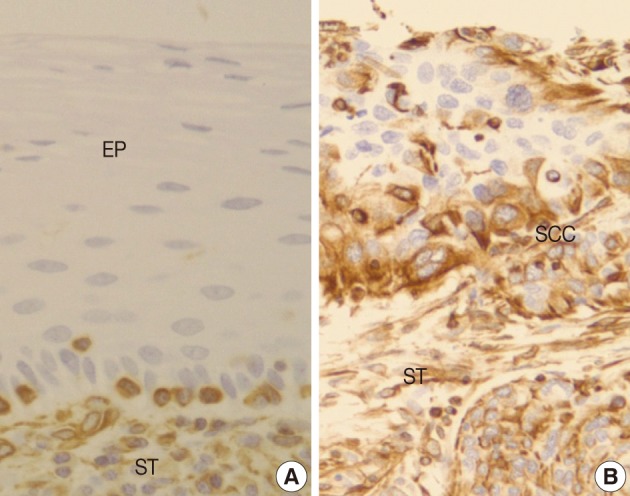
Vimentin
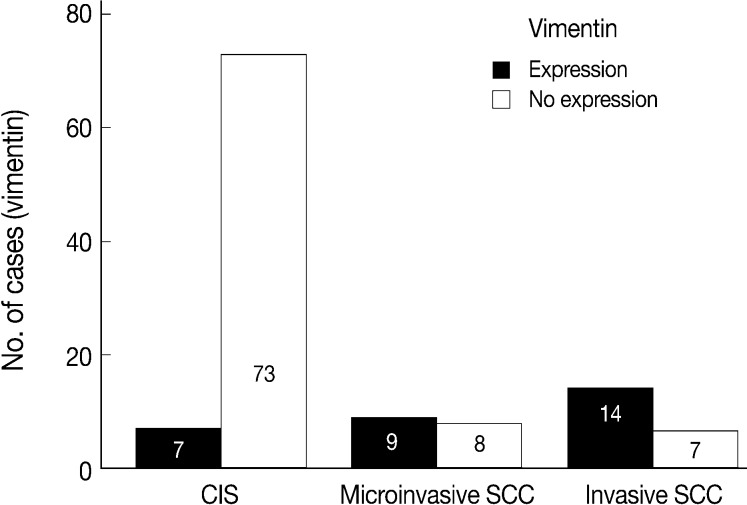
Normal squamous mucosa showed no immunoreactivity for vimentin and there were only some scattered mesenchymal cells within it, which were considered to be entrapped (Fig. 4A). However, squamous carcinoma cells revealed a relatively dense cytoplasmic expression for vimentin, showing polygonal or multipolar appearance and heterogeneously scattered pattern (Fig. 4B). Additionally, there was no big difference in the staining pattern between non-invasive and invasive foci of the CIS and microinvasive (or invasive) carcinoma groups.
Comparison of immunoexpressions for EGFR, Snail-1, E-cadherin, and vimentin between CIS, microinvasive, and invasive SCC groups
EGFR overexpression was found in 70.4%, 82.4%, and 71.4% of CIS, microinvasive, and invasive SCC groups, respectively. There was no difference in EGFR expression rates between three groups (Fig. 5). Snail-1 was expressed more frequently in the nuclei of the tumor cells and in the underlying stromal tissue than in the normal control. Snail-1 was expressed in 82.7%, 82.4%, and 81.0% of CIS, microinvasive and invasive groups, respectively. Thus, no significant difference in Snail-1 expression was found between them (data not shown). When E-cadherin expression was classified as three patterns such as S, W&H, and HEG ones, W&H and HEG patterns were considered to show either a loss of or reduced E-cadherin expression. CIS group predominantly revealed 70% cases of strong or memebranous pattern of E-cadherin expression, whereas the invasive and microinvasive SCC groups only revealed 11.8% and 23.8%, respectively. The correlation between the loss of E-cadherin and stromal invasion was statistically significant (p<0.05) (Fig. 6). Moreover, when E-cadherin expression was analyzed by the pattern of either membranous or cytoplasmic staining, increased cytoplasmic immunoreactivity or loss of membranous immunoreactivity was found significantly higher in invasive SCC cases than in CIS group (p<0.001) (Fig. 7). Vimentin, a representative mesenchymal cell marker, was immunoexpressed aberrantly (>5% of entire cells) in 60.5% and 8.8% of the invasive and CIS cases, respectively. Meanwhile, normal and metaplastic squamous epithelium revealed none or rare (<5%) immunoreactive cells in the normal control tissues. Thus, microinvasive and invasive SCC cases showed a much higher vimentin expression than the CIS group (p<0.001) (Fig. 8).
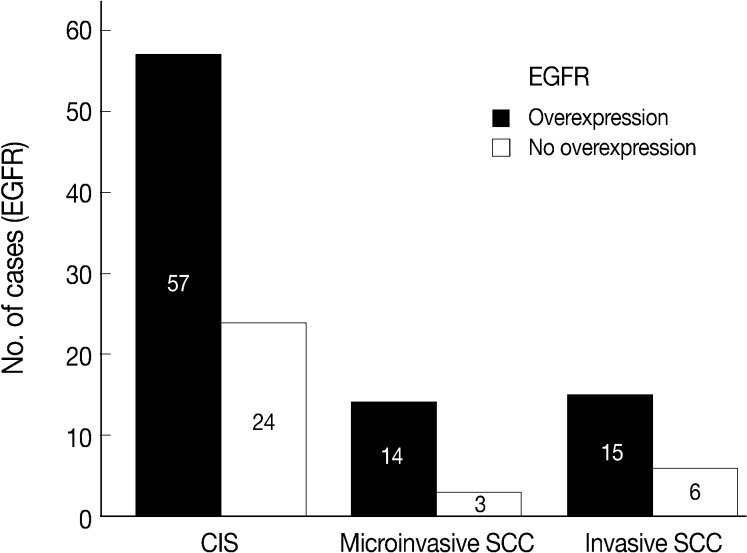
A chart comparing epidermal growth factor receptor (EGFR) overexpression between carcinoma in situ (CIS), microinvasive, and invasive squamous carcinomas reveals no significant difference among them (p>0.05). Numbers in bars, numbers of the immunostained cases; SCC, squamous cell carcinoma.

Comparison of E-cadherin expression patterns between carcinoma in situ (CIS), microinvasive, and invasive squamous cell carcinomas (SCCs) reveals a marked difference in E-cadherin expression of strong or membranous pattern which is significantly reduced in the invasive carcinoma group (p<0.05).
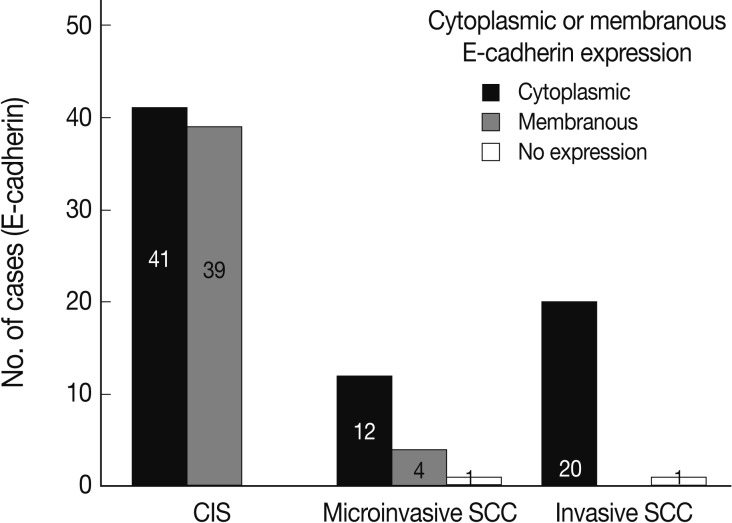
E-cadherin expression pattern analyzed by either membranous or cytoplasmic staining reveals significantly increased cytoplasmic immunoreactivity or loss of membranous immunoreactivity in the invasive squamous cell carcinomas (SCCs) compared to the carcinoma in situ (CIS) group (p<0.05).
Inter-relationship between immunoexpressions for EGFR, Snail-1, E-cadherin, and vimentin as EMT markers in CIS and invasive SCC groups
In CIS group alone, there was a significant correlation between EGFR overexpression and Snail-1 upregulation. EGFR-positive cases showed a significantly higher Snail-1 expression (89%) than the EGFR-negative ones (67%; p=0.022) (Table 1).
In invasive SCC group including microinvasive carcinoma cases, there were statistically significant interrelationships between E-cadherin and Snail-1 and between vimentin and Snail-1. Reduced expression patterns (W&H and HEG) of E-cadherin were more frequent in cases showing Snail-1 upregulation than in cases without Snail-1 upregulation (p=0.026) (Table 2). Furthermore, the invasive carcinomas with Snail-1 upregulation showed much higher vimentin expression (71%) than those without Snail-1 upregulation (14%; p=0.010) (Table 3).
Interrelationship between E-cadherin and Snail-1 in 38 cases of microinvasive and invasive squamous carcinomas
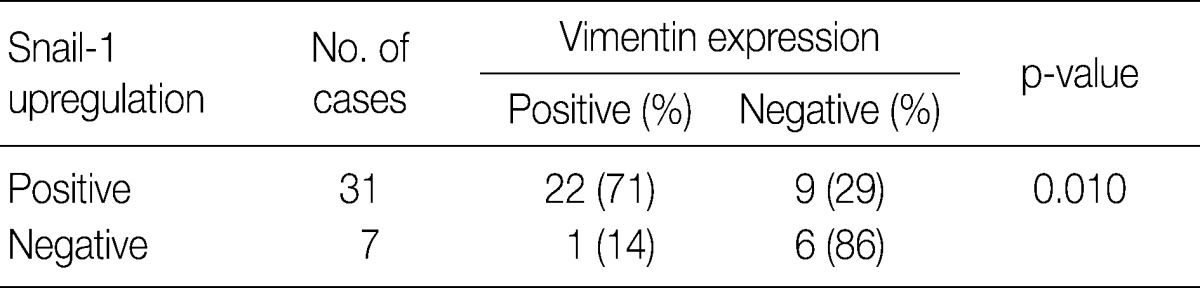
Interrelationship between Snail-1 and vimentin in 38 cases of microinvasive and invasive squamous carcinomas
In overall 118 cases including 80 CIS and 38 invasive SCC cases, there was an inverse relationship between E-cadherin and vimentin expressions, because the overall cases with reduced E-cadherin expression disclosed a significantly higher vimentin expression (45%) than the cases with strong membranous E-cadherin expression (10%; p<0.001) (Table 4).
DISCUSSION
Stromal invasion of the tumor cells has been known to be an important criterion for malignancy and is also applied frequently to distinguish the invasive cervix cancer from squamous cell CIS. It is thought to be associated with EMT, which is characterized by the change of the phenotypes from epithelial cells to mesenchymal cells, disassembly of intercellular junctions, and increased cell motility. A recent study has concluded that EGF is a novel EMT inducer in cervical cancer cells based on several in vivo and in vitro results.1 Their conclusion was supported by the following results: a) Chronic EGF treatment induced elongation of cell shape, increased cell scattering and enhanced cancer cell invasion; b) EGF caused decreases in epithelial markers such as E-cadherin and β-catenin; c) EGF caused increases in mesenchymal markers such as vimentin, α-smooth muscle actin, and fibronectin. Their study has also demonstrated that cervical carcinoma progression is accompanied by overexpressed EGFR in parallel with downregulated E-cadherin and upregulated vimentin. In general, downregulation of E-cadherin has been known as the key step for the invasive phase of carcinoma, and its dominant transcriptional repression has been reported to be largely responsible for the loss of E-cadherin expression.9,10 Recent evidences have shown that Snail-1 plays a fundamental role in EMT through its suppression of E-cadherin, and it also induces upregulation and redistribution of mesenchymal markers such as vimentin and fibronectin.5,11 In normal cervical mucosa, EGFR is found to be in the cytoplasm and the membrane of the cells within the basal layer and its expression gets increased in the cytoplasm by HPV infection and associated with the increasing grade of intraepithelial neoplasia.7,12 In our study, both membranous and cytoplasmic immunostainability for EGFR was found to be increased in the tumor tissues compared to the internal control tissue, but there was no significant difference between the CIS and the SCC groups. However, 81 cases of CIS group showed a positive correlation between EGFR and Snail-1 immunoexpressions (p=0.022). Snail-1, which is another potent EMT inducer, suppresses E-cadherin expression and further controls the proteolytic activity of the matrix metalloproteinases that contribute to the phenotypic changes associated with EMT and stromal invasion.13,14 With EGFR activation, Snail-1 is then upregulated, accumulates in the nuclei, and performs its transcriptional function on EMT markers.1 In the present study, there was no significant difference in Snail-1 immunoexpressions between CIS and the invasive SCC groups, although invasive SCC cases revealed higher Snail-1 immunoexpression in the vimentin-positive and E-cadherin-negative cases than in the vimentin-negative and E-cadherin-positive ones (p=0.010 and p=0.026, respectively). These results might suggest that the upregulated Snail-1 protein in the invasive SCC plays a key role in stromal invasion by increasing the discohesiveness of tumor cells and acquisition of vimentin expression.
In a similar-sized study (121 cases), high-grade squamous intraepithelial lesion (HSIL) and invasive cases showed significantly reduced E-cadherin expression and increased cytoplasmic immunoreactivity compared to the low-grade SIL (LSIL), atypical squamous cell of undetermined significance, and normal groups.15 They concluded that decreased E-cadherin expression appears to be a useful parameter of the malignant potential of cervical lesions. However, they didn't compare it between CIS and microinvasive SCC, although CIS is an important lesion with a potential of impending stromal invasion. The present study is, therefore, thought to be the first to demonstrate that E-cadherin might be the best indicator of stromal invasion of the CIS lesions, in that microinvasive and invasive SCC cases showed a significantly reduced or loss of E-cadherin expression compared to the CIS group (p<0.001). A loss of membranous staining and a progressive increase in cytoplasmic staining of E-cadherin have been reported in several studies to be observed from LSILs to HSILs to invasive SCC.15-17 In our study, this tendency for E-cadherin immunoreactivity to be increased in the cytoplasm or lost in the cell membranes was also observed from CIS, microinvasive, and invasive SCC by either cytoplasmic (51%, 71%, and 95%, respectively) or membranous (49%, 23%, and 0%, respectively) immunoreactivities. The differences in the immunoreactivities were statistically significant between CIS and microinvasive or invasive carcinomas (p<0.001). The cytoplasmic staining and loss of membranous E-cadherin observed in the HSIL and invasive SCC is likely to reflect a nonfunctional protein form of E-cadherin since it should be present at the cell membrane in order to perform its integral roles for intercellular cohesion and epithelial tissue integrity. The nonfunctional cytoplasmic E-cadherin may indicate an increased rate of protein synthesis and cell proliferation and consequently the molecule en route from the endoplasmic reticulum to the cell surface membrane, or failure of the protein to translocate or attach to the cell membrane due to the absence of other associated proteins.18-20 According to our results, higher cytoplasmic E-cadherin immunopositivity and more marked loss of membranous staining pattern in the microinvasive and invasive carcinomas than in CIS lesions appeared to be the indicator of the progression of CIS into microinvasive or invasive carcinoma, especially when there are suspicious foci of stromal invasion in any CIS lesions (Fig. 3).
There is few data about vimentin expression as an EMT marker in cervical cancer progression. A recent study on EMT in cervical cancer tissue and cultured cells demonstrated vimentin expression by an immunofluorescent staining technique in the invasive or metastatic tumor cell nests, with no or rare expression in the normal or superficial (noninvasive) tumor cell nests.1 They have concluded that the progression of cervical carcinoma is accompanied by an upregulation of vimentin. However, data on the comparison of the vimentin expression profiles between squamous intraepithelial lesions and invasive carcinomas were not available and thus its role in the EMT process could not be elucidated in their study. In the present study, normal control tissues immunohistochemically presented almost no vimentin expression and the CIS lesions showed less than 10% of immunoreactivity (8.8%). Meanwhile, microinvasive and invasive carcinomas showed a much higher vimentin expression with 53% and 67% cases, respectively. Thus, it is considered that the significantly increased vimentin expression could be used as an important EMT marker in the progression of CIS into microinvasive or invasive SCC in the human cervical tissues.
In summary, we obtained the necessary results for elucidating the important EMT markers playing in the stromal invasion of CIS as follows:
Of the four EMT markers, reduced E-cadherin expression and acquisition of vimentin expression were found to be significantly higher in the invasive than in the CIS group.
CIS group revealed a positive correlation between EGFR and Snail-1 expressions.
In the invasive carcinoma group, upregulated Snail-1 protein was correlated with loss of E-cadherin expression and gain of vimentin expression.
Overall group (119 cases) exhibited a statistically significant inverse correlation between E-cadherin and vimentin immunoexpressions.
Based on these summarized results, we could arrive at a conclusion that loss of E-cadherin and acquisition of vimentin expression in the CIS of human uterine cervix might be noble indicators of the progression of CIS into microinvasive and subsequently into invasive SCC via EMT. However, in the present study, no significant difference in EGFR or Snail-1 protein expression between CIS and invasive SCC was demonstrated during the EMT process. Thus, further studies are needed to elucidate control mechanisms, other than EGFR overexpression and Snail-1 upregulation, in the cervix cancer progression.
Acknowledgments
The present research was conducted by the research fund of Dankook University in 2010.
Notes
No potential conflict of interest relevant to this article was reported.