Endoscopic Ultrasound-Guided Fine Needle Aspiration Cytology Diagnosis of Solid Pseudopapillary Neoplasm: Three Case Reports with Review of Literature
Article information
Abstract
Solid pseudopapillary neoplasm of the pancreas (SPN) is relatively rare and it occurs almost exclusively in women. We recently experienced three cases of SPN diagnosed by endoscopic ultrasound-guided fine-needle aspiration (EUS-FNA). These three cases were two male and one female patient whose age was 29, 37, and 44 years old. Radiological diagnosis was pancreatic endocrine tumor (PEN) showing solid with a heterogenous echogenicity. EUS-FNA cytology specimens consisted of single cells and aggregates of uniform cells, forming microadenoid structures, branching, papillary clusters with delicate fibrovascular cores. In conclusion, a single diagnosis of SPN based on clinical and radiological findings would be risky because there is a possibility of it being misdiagnosed as PEN or other malignancies. An EUS-FNA is therefore essential for establishing the diagnosis. In addition, the pathologists should recognize the characteristic cytologic findings with immunoprofiles of SPN to prevent misdiagnosis of SPN.
Solid pseudopapillary tumors of the pancreas (SPN) constitute approximately 1-2% of all exocrine pancreatic tumors.1 They are rare, indolent, epithelial neoplasms which frequently undergo hemorrhagic necrosis and subsequent cyst formation. In addition, they show a female predilection; 90% of them occur predominantly in young women with a mean age 35 years. But they rarely cause symptoms, and can be located anywhere in the pancreas.2 SPN are usually discovered incidentally on computed tomography (CT) or magnetic resonance imaging (MRI) scans and the recommended treatment is a surgical resection because they have a malignant potential. Traditionally, percutaneous fine-needle aspiration (FNA) has been a method for making a preoperative diagnosis of them.3,4
According to recent studies, a small series of patients could be diagnosed with solid pseudopapillary tumor on endoscopic ultrasound-guided FNA (EUS-FNA).5,6 It is a highly-sensitive, specific diagnostic modality for lesions of the pancreas as well as adjacent organ sites.7 Its high degree of accuracy is achieved through understanding the key cytologic features of the various pancreatic lesions. This has been previously documented in the literature.8,9 SPN are pancreatic neoplasms whose cytologic features have been well described.5,10,11 Nevertheless, SPN continue to pose significant diagnostic challenges even for experienced cytopathologists and often unsuspecting ones.
We experienced three cases of SPN diagnosed on the EUS-FNA. Our cases highlight the importance of cytopathologic features in making a differential diagnosis between SPN and its closest morphologic mimicker, pancreatic endocrine neoplasm (PEN). Here, we report our cases with a review of literatures.
CASE REPORTS
Clinical findings
Case 1
A 44-year-old man with no previous medical history presented to the hospital complaining of the right lower quadrant abdominal pain. The patient had no history of injury, melena, fever, hematochezia, nausea or vomiting. On ultrasonography and CT scans of the abdomen, there was a pancreatic head mass (Fig. 1A). The patient was therefore transferred to our hospital for further evaluation and treatment. The patient underwent EUS. This showed that the patient had a well-circumscribed, 4 cm-sized heterogeneous echogenic mass in the pancreatic head (Fig. 1B). Then, the patient underwent EUS-FNA, whose findings were initially suggestive of PEN. The patient underwent pylorus preserving pancreatico-duodenectomy but received no further treatment. At the time of writing, the patient had no evidence of residual tumor.
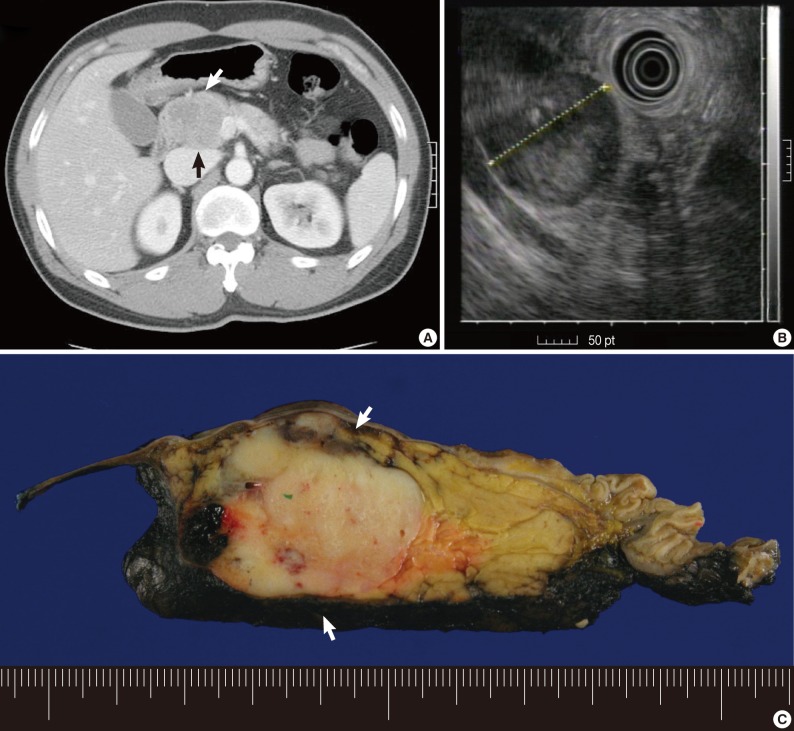
(A) A well-defined, partly enhancing 4.0 cm-sized mass is present in the pancreatic head of case 1 (arrows). (B) An endoscopic ultrasound image shows a 4.0 cm-sized, round, well-defined and heterogeneously hypoechoic solid mass in the pancreatic head. (C) A whitish-tan, solid mass with hemorrhage is noted in the pancreatic head on the resected specimen (arrows).
Case 2
A 37-year-old previously healthy woman incidentally had a 1.7 cm-sized pancreatic mass detected on abdominal ultrasonography during the regular medical check-up. The patient was transferred to us for further evaluation and treatment. On abdominal CT scans, there were findings that are suggestive of a small, low-attenuated mass in the pancreatic body with a minimal dilatation of the distal pancreatic duct. Under the clinical impression of pancreatic cancer, the patient underwent EUS for further evaluation of the pancreatic lesion. This showed that the patient had a well-defined, homogeneously echogenic mass in the pancreatic body. The patient underwent EUS-FNA followed by the distal pancreatectomy without adjuvant chemotherapy.
Case 3
A 29-year-old previously healthy man presented to the hospital complaining of dyspepsia and constipation. In spite of the conservative medication, the patient had no symptoms improved. On abdominal CT scans, there was a benign-looking, 5 cm-sized mass in the pancreatic tail. The impression was a microcystic serous cystadenoma or a pancreatic cancer, for which the EUS was performed. Then, the EUS showed that there was a well-circumscribed, homogeneous echogenic mass with a mostly solid containing calcification. This was followed by the FNA. Following this, the patient underwent spleen-preserving distal pancreatectomy.
Pathologic findings
FNA findings
The smears were highly cellular with a population of small, uniform cells in cohesive, often branching and papillary cell clusters, or interspersed with many single cells. The background was clean or filled with hemorrhage. Delicate fibrovascular cores with myxoid stroma were noted. Because cell clusters were admixed with hemorrhage, characteristic architectures were not clearly indentified in case 1, which lead to misdiagnosis of PEN. Individual tumor cells are uniform with round-to-oval nuclei, smooth to slightly indented or grooved nuclear membranes, even finely granular chromatin and inconspicuous nucleoli (Fig. 2A). The cytoplasm is scanty to moderate. Some tumor cells show rosette formation with luminal myxoid globule (Fig. 2B).
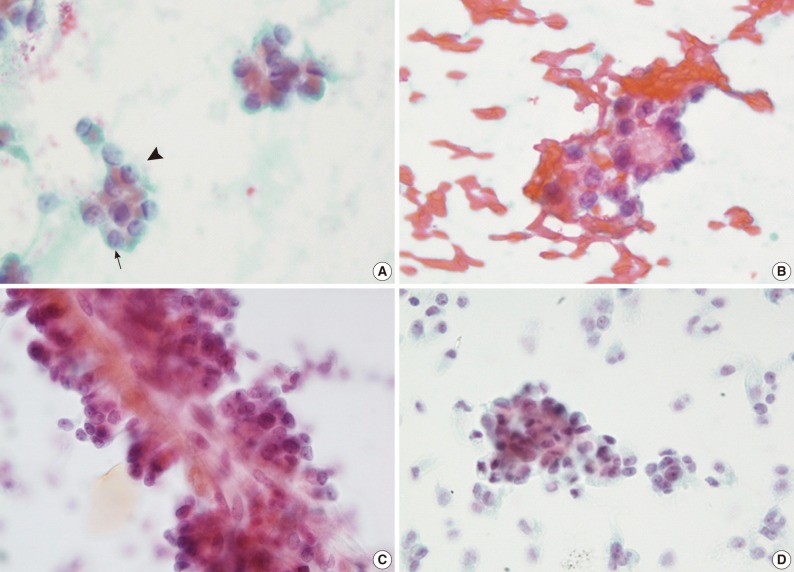
(A) In case 1, there are inconspicuous nucleoli (arrowhead) and nuclear grooves (arrow). (B) In case 2, the tumor cells show a rosette formation with a myxoid globule in the background of hemorrhage. (C) Case 3 is characterized by the papillary arrangement composed of delicate fibrovascular core with attached multilayer monotonous cuboidal cells. (D) In case 3, both a cluster of tumor cells and scattered ones are identically showing relatively uniform, monotonous features.
Liquid-based cytology smear was performed in one patient (case 2). The cytologic findings were similar to those of conventional smears. In comparison with conventional smear, however, papillary cell clusters were more frequent and more easily detected (Fig. 2C) in clean background without hemorrhage (Fig. 2D). Individual cells showed finely granular chromatin with more prominent nucleoli. There are no differences in the indentation or nuclear grooves between the two methods.
Gross findings
All tumors were well-circumscribed and demarcated from the pancreatic tissue without capsule. In case 1, the tumor was 4 cm in size and located in the pancreatic head and its cut surface was grayish-white, solid with focal hemorrhage. No cystic change was identified (Fig. 1C). In case 2, the tumor was 1.7 cm in size and located in the pancreatic body. The tumor was grayish tan, solid with hemorrhage in 20% of tumor volume. No cystic changes or capsule were noted. In case 3, there was a 5 cm-sized, well-demarcated solid mass in the pancreatic tail. The mass showed a hemorrhage without cystic change.
Microscopic findings
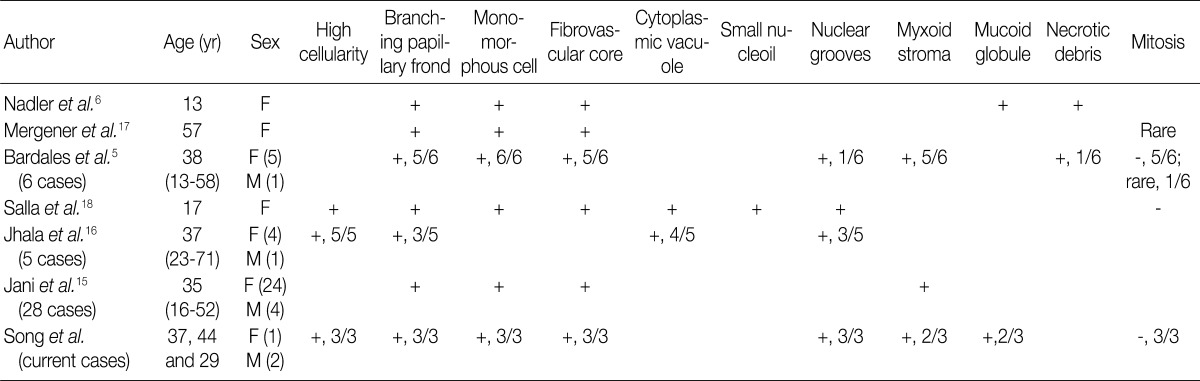
All tumors were composed of sheets of relatively uniform, loosely cohesive or distracted cells with eosinophilic slightly granular cytoplasm and uniform nuclei with nuclear grooves. There were delicate background vasculatures (Fig. 3A). By this dyscohesive nature of tumor cells, cells were loosely adherent to the blood vessels, forming characteristic pseudopapillae. Cores of pseudopapillae stroma or either myxoid or hyalinized (Fig. 3B and 3C). Foam cells and red blood cells were scattered among the neoplastic cells. Some of the neoplastic cells had not only a clear, almost vacuolated cytoplasm but also intracytoplasmic eosinophilic globules. But there was a lack of mitotic figures. Immunohistochemical staining was performed in all the three cases (Fig. 4), whose results are summarized in Table 1.
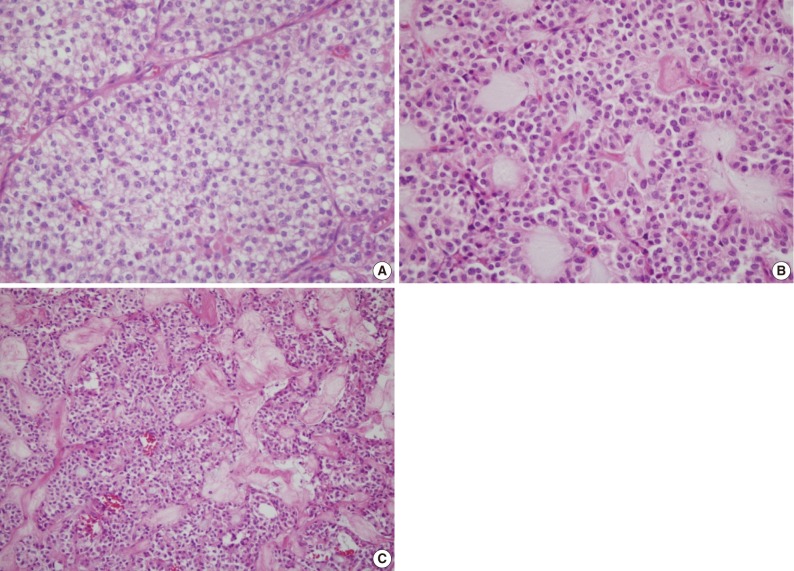
(A) Some areas of tumor show a solid sheet with thin fibrovascular cores and clear or eosinophilic granular cytoplasm. Intraluminal myxoid globules (B) and eosinophilic hyalinized stroma (C) are frequently observed.
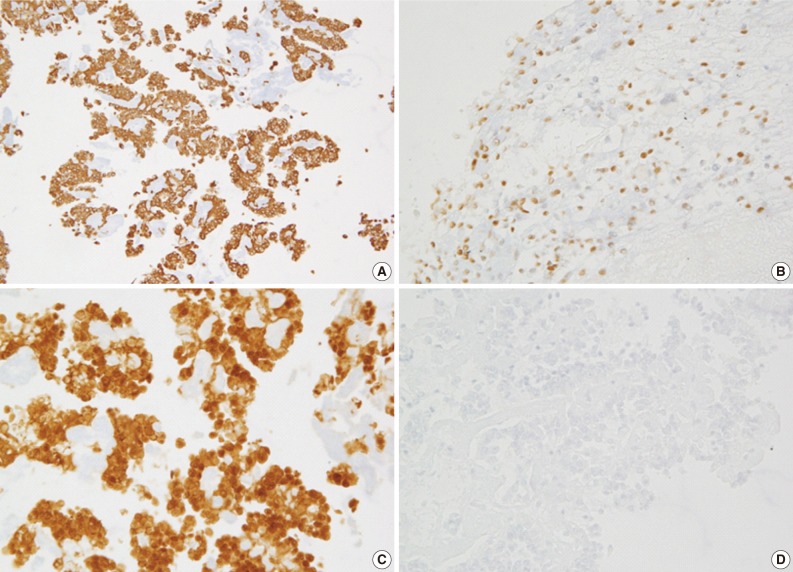
In case 3, the tumor cells show an immunopositivity for vimentin (A), progesterone receptor (B), and β-catenin (C) and an immunoreactivity for a loss of E-cadherin (D).
DISCUSSION
Diagnosis of SPN is important for the clinical management of patients. Diagnostic modalities including CT and MRI can only suggest a diagnosis of SPN. CT findings include an encapsulated lesion with a well-defined margin and variable central areas with cystic degeneration, necrosis or hemorrhage. Calcifications may occasionally be seen. MRI is helpful for identifying the characteristic internal signal intensities of blood products, which is useful in making a differential diagnosis of SPN from other cystic pancreatic tumors.12 Advances in technology have permitted the performance of FNA biopsy under EUS guidance.13
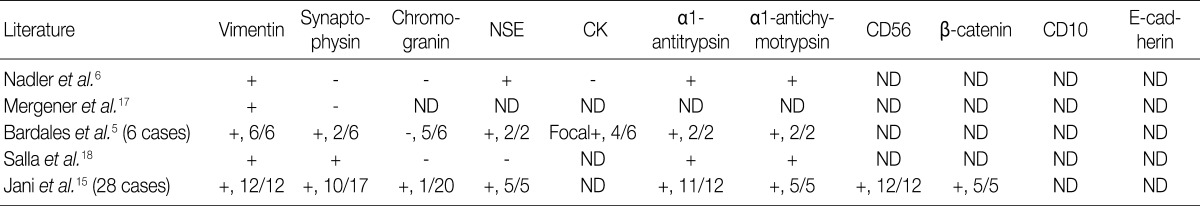
EUS permits a better evaluation of SPN, but the findings also are not specific. Because SPN occurs almost exclusively in women, with an approximate female-to-male ratio of 9:1, it might be difficult to make a preoperative diagnosis if the case presents with unusual clinical and radiologic findings for which an accurate preoperative diagnosis using EUS-FNA cytology would be highly desirable. This is because a local surgical excision is usually curative in SPN. Since Bondeson et al.14 first made a correct diagnosis of SPN on the preoperative FNA, 57 cases of SPN have been diagnosed based on cytologic findings on the percutaneous FNA.5,10,11,14 Recently, some cases of SPN have also been diagnosed on the EUS-FNA.5,6,15-18 We summarized EUS-FNA cytologic features of SPN (Table 2) and their immunohistochemical profiles (Table 3) according to a review of the English literatures. FNA cytomorphologic features are highly characteristic and distinct from those of other cystic or solid tumors of the pancreas. On aspirated materials, the most frequent features are the presence of marked cellularity with pseudopapillary fragments composed of fibrovascular cores lined with one to several layers of tumor cells intermingled with discohesive neoplastic cells.5,6,15-18 As shown in our cases, inter- or intra-cellular pink hyaline globules, mucus-like globules are surrounded by the stromal cells and cellular debris, which is also one of the frequent features.
Histologic differential diagnosis of SPN from PEN or pancreatic adenocarcinoma is important. This is because SPN have a much better prognosis compared with PEN or pancreatic adenocarcinoma, with only 10% to 15% of cases recurring or metastasizing. More than 95% of SPN are cured by complete surgical resection alone.19 The uniform, bland-appearing, often dispersed epithelial cells of SPN can greatly resemble those of PEN. When the papillary architecture is absent in SPN cytologic specimens, a careful attention to the nuclear details that are commonly seen in many cases of SPN, including the presence of nuclear grooves, indentations, the occasional perinuclear vacuole or intracytoplasmic hyaline droplet, will be useful in making a differential diagnosis between the two disease entities. In an actual clinical setting, however, it would be almost impossible to make a differential diagnosis between the two disease entities without an ancillary test. In case 1, it was particularly difficult to make a diagnosis of SPN because the tumor cells were arranged in solid nests and pseudopapillary architectures were not prominent. The possibility of PEN could not be completely ruled out even in the resected specimens. We could therefore make a diagnosis based on immunohistochemical stains.
The aspirated tissue from any lesion can be prepared in a number of different ways: direct smears, cytospins, liquid-based preparations and cell blocks. Direct smears are the most common, particularly for solid masses, but specimens aspirated as a fluid from cyst are often processed as cytospins or liquid-based specimen. Liquid-based preparations eliminate obscuring blood and inflammation and thereby provide excellent cellular preservation. In case 3, the aspirated prepared in a liquid-based manner, the papillary branching structures were well preserved with a more prominent fine granular chromatin and small nucleoli because the blood was eliminated. In addition, cell block preparation is advantageous given the readily available tissue for immunocytochemistry.
Immunohistochemically, most cases of SPN are immunoreactive for vimentin, α1-antitrypsin and α1-antichymotrypsin; occasionally positive for neuron specific enolase and synaptophysin; and non-reactive for carbohydrate antigen 19.9 and chromogranin A.5,6,15-18 Recently, Kim et al.20 have reported that a loss of E-cadherin and the cytopalsmic-nuclear expression of β-catenin are the immunological profile that is the most useful in making a diagnosis of SPN. Because there was a possibility of case 1 being PEN based on the absence of papillary configuration and an unusual clinical setting, we performed an immunohistochemistry for only two antibodies, thus causing a misdiagnosis. The tumor cells in cell block were immuno-positive for synaptophysin and immuno-negative for chromogranin, based on which case 1 was initially diagnosed with PEN. After the resection of the pancreas mass, the diagnosis was revised to SPN with following immune-profiles: vimentin (+), progesterone receptor (+), CD10 (+), β-catenin (+) and E-cadherin (-). Then, we could make an accurate diagnosis of other two cases on immunohistochemistry of cell blocks. It can therefore be inferred that immunohistochemical stains based on cell block is as important as cytologic features in making an accurate diagnosis of pancreatic neoplasms.
In conclusion, our cases indicate that the cytomorphologic features from EUS-FNA, as well as the clinical correlation and radiological findings, are essential for making an accurate diagnosis of SPN. Therefore, the pathologists should recognize the importance of the characteristic cytologic findings with immunoprofiles of SPN to prevent misdiagnosis of SPN.
Notes
No potential conflict of interest relevant to this article was reported.