Cytologic Features of Giant Cell Ependymoma: A Case Report and Review of the Literature
Article information
Abstract
Here, we present a case of anaplastic giant cell ependymoma (GCE) occurring in a 15-year-old woman. Squash smear slides for intraoperative frozen section diagnosis revealed oval to round cell clusters with a papillary structure in a fibrillary background. This was occasionally accompanied by the presence of bizarre pleomorphic giant cells with hyperchromatic nuclei and prominent intranuclear inclusions. These intranuclear inclusions were a key clue to diagnosis of ependymoma. Histologic analysis revealed features of a high-grade tumor with perivascular pseudorosettes and bizarre pleomorphic giant cells, which established the diagnosis of GCE. We performed a review of literatures about the cytologic features of GCE, including our case, thus proposing that intraoperative frozen diagnosis of GCE would be established by squash smear preparations featuring the mitosis and necrosis, as well as the high cellularity, and the presence of giant cells showing hyperchromatic nuclei with eosinophilic cytoplasm and intranuclear inclusions/pseudoinclusions.
Ependymoma is a slowly-growing tumor composed of neoplastic ependymal cells, and it accounts for 2-9% of all neuroepithelial tumors. Histologically, the classic pattern of ependymoma is characterized by the presence of round-to-oval nuclei with "salt and pepper" speckling of the chromatin. The presence of perivascular pseudorosettes and ependymal rosettes is a key histologic feature of the diagnosis. Apart from this classic pattern of ependymoma, giant cell ependymoma (GCE) is described in the World Health Organization (WHO) classification as a histopathological variant of ependymoma.1
Since Zec et al.2 first reported two cases of GCE in 1996, several cases of GCE have been reported in the literature. They are summarized in Table 12-13 along with the present case. There is a variability in the tumor site and the age distribution in patients with GCE. Despite of the presence of pleomorphic giant cells, GCE has a relatively good prognosis. Due to a small number of reported cases, however, it is difficult to define its characteristics.
Here, we report a case of GCE arising from the temporooccipital area in a 15-year-old woman and with a review of literatures focusing on its cytologic features.
CASE REPORT
A healthy 15-year-old woman was admitted to the department of neurosurgery of our institution with a chief complaint of a 1-month history of headache and dizziness. Magnetic resonance imaging (MRI) (Fig. 1) demonstrated a 6.8×5 cm-sized, solid and cystic intra-axial mass in the right temporooccipital area, compressing the posterior horn of the right lateral ventricle. The solid portion of the mass included a calcification, thus presumably partly infiltrating into the brain parenchyma. This was accompaned by the presence of edema of the adjacent brain parenchyma. Differential diagnoses based on radiology include astroblastoma, ependymoma, pleomorphic xanthoastrocytoma and supratentorial primitive neuroectodermal tumor.

Preoperative magnetic resonance imaging scans. A 6.8×5 cm-sized solid and cystic intra-axial mass is present in the right temporooccipital area, compressing the posterior horn of the right lateral ventricle. (A) Transverse and (B) sagittal views.
The patient underwent a craniotomy. Thus, grossly, the supratentorial tumor was completely resected. The surgeon noted that the tumor was highly vascularized. Postoperatively, the patient received a focal fractionated radiotherapy with a total dose of 5,040 cGy. A follow-up MRI was taken on postoperative month 5, which revealed no recurrence or progression of the tumor.
For intraoperative frozen diagnosis, we used the square-smear technique (Fig. 2). This revealed a hypercellular smear in a fibrillary background. Most of the small- to medium-sized cells with papillary structures had hyperchromatic nuclei and coarse chromatin. This was occasionally accompanied by the presence of bizarre pleomorphic giant cells. They had a round-to-oval shape and contained hyperchromatic nuclei, eosinophilic cytoplasm and prominent eosinophilic intranuclear inclusions. These intranuclear inclusions were a key clue to differential diagnosis of ependymoma and meningioma. Considering the cytologic features along with the clinical and radiological data, we made an intraoperative frozen diagnosis of ependymoma. With a retrospective review of the slides, we identified a perivasculasr pseudorosettes-like lesion. Thus, we supported a frozen diagnosis of ependymoma.
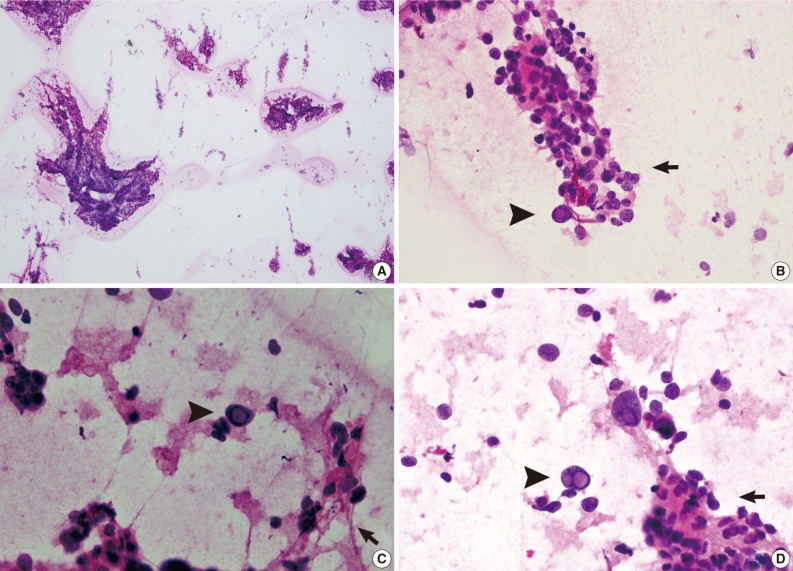
Hematoxylin and eosin-stained squash slides for frozen diagnosis show a hypercellular smear (A) in a fibrillary background (C, sharp arrow). Multiple pleomorphic giant cells with hyperchromatic nuclei and eosinophilic cytoplasm are seen. Eosinophilic intranuclear inclusions are prominent (B-D, arrowheads). Perivascular pseudorosettes-like lesions are also noted (B, D, arrows).
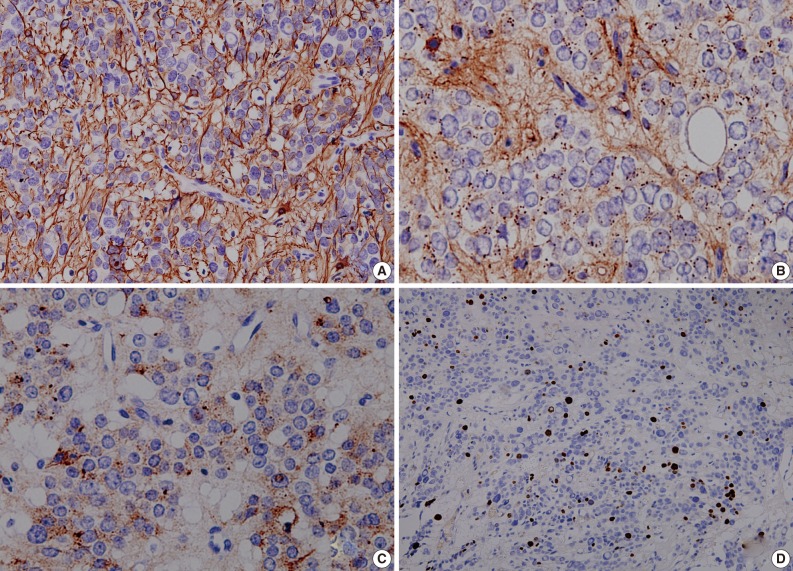
The surgical specimens consisted of multiple pieces of soft red-to-grey tissue. They were fixed with a 10% buffered formalin and then paraffin-embedded. Then, the specimens were sectioned at a thickness of 2 µm and then stained with a hematoxylin and eosin dye. For immunohistochemistry, we used antibodies against glial fibrillary acidic protein (GFAP), epithelial membrane antigen (EMA), cytokeratin, Ki-67, synaptophysin, and CD99 (MIC2).
As shown in Fig. 3, a histopathologic examination showed that the tumor had perivascular pseudorosettes; this is one of the characteristic features of ependymoma. The tumor cells had histopathological findings that are consistent with squash smear ones described above. This was also accompanied by the frequent presence of bizarre pleomorphic giant cells with prominent intranuclear eosinophilic inclusions. According to the WHO criteria, it had features of an anaplastic tumor, including a marked cellularity, abundant mitoses, vascular proliferation and necrosis. Immunohistochemically, the tumor showed a diffuse expression of both GFAP and synaptophysin. That is, it had a high intensity, a dot-like expression of CD99 and that of EMA. In addition, the tumor cells had a Ki-67 labeling index of about 10% (Fig. 4). Based on all of these findings, a diagnosis of anaplastic GCE was established.
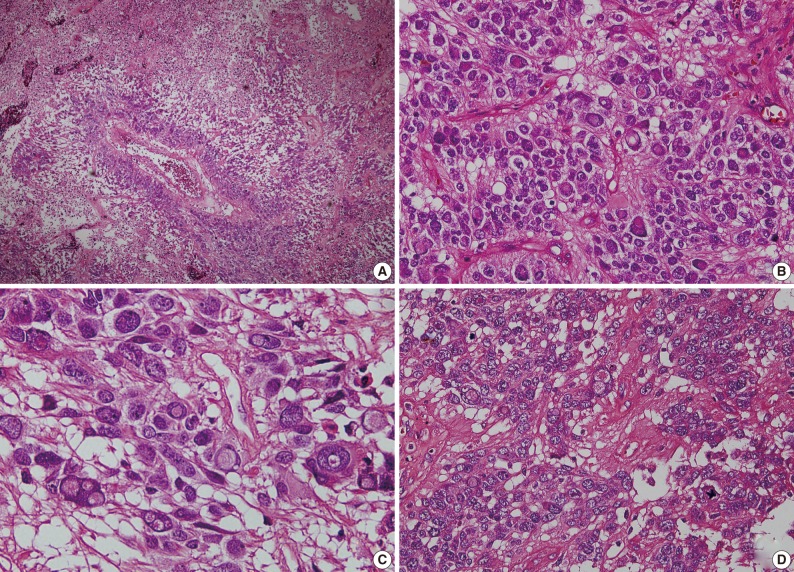
Hematoxylin and eosin-stained permanent tissue sections show perivascular pseudorosettes and geographic necrosis (A), bizarre pleomorphic giant cells with prominent intranuclear eosinophilic inclusions (B, C) and mitosis (D).
DISCUSSION
A rare variant of ependymoma, GCE poses a diagnostic challenge for the pathologists on the intraoperative frozen section as well as the permanent section. The presence of perivascular pseudorosettes is a key histologic clue to diagnosis of ependymoma. But pseudorosettes are not present in all the case of GCE. According to Zec et al.,2 who first described two cases of GCE in 1996, the absence of perivascular pseudorosettes in GCE might reflect the failure of the neoplastic cells to elaborate perivascular process. Moreover, perivascular pseudorosettes cannot be easily found on the intraoperative frozen section. This often leads to the misdiagnosis of GCE as glioblastoma multiforme,11 anaplastic astrocytoma,6 subependymomal giant cell astrocytoma or tanycytic ependymoma5 on intraoperative frozen section. In addition, GCE should also be differentially diagnosed from anaplastic oligodendroglioma, clear cell ependymoma, pleomorphic xanthoastrocytoma and giant cell glioblastoma.10
Despite these diagnostic challenges, there has been an increase in the demand for rapid intraoperative diagnosis. This is particularly case with the neurosurgical practice. A simple, reliable, and rapid method, the squash smear technique is useful to present detailed cytologic features of lesions. It is useful in making an intraoperative diagnosis of central nervous system lesions.14 To our knowledge, however, there are no reports about the cytologic features of GCE.
We performed a review of literatures about GCE, focusing on the cytologic features seen on the tissue sections, whose results including our case are summarized in Table 2. The cytologic features are classified based on the cellularity, hyperchromatic nuclei, binucleation or multinucleation, eosinophilic cytoplasm, intranuclear inclusion/pseudoinclusions, perivascular pseudorosettes, brisk mitosis, necrosis and fibrillary background. Basically, all the 14 cases showed hypercellularity, mitosis and necrosis. Of the total cases, 93% (13/14) had eosinophilic cytoplasm and perivascular pseudorosettes; 71% (10/14) did hyperchromatic nuclei; 57% (8/14) did intranuclear inclusions/pseudo-inclusions; and 50% (7/14) did binucleation or multinucleation.
The cytologic features of GEC are described in Table 2. It is noteworthy, however, that these features are based on tissue sections of GCE rather than cytology specimens such as the squash smear preparations. It is, therefore, a matter of course that there is no consistency in the cytologic features between the tissue sections and the cytology specimens. In our case, there were perivascular pseudorosettes on the tissue sections, but not found on the squash smear preparations. But both diagnostic modalities showed such findings as mitosis and necrosis, giant cells and intranuclear inclusions/pseudoinclusions. Further comparative descriptions are warranted to define the cytologic features of GCE between tissue sections and cytology specimens.
In making an intraoperative frozen diagnosis based on squash smear preparations featuring the mitosis and necrosis, as well as the high cellularity, and the presence of giant cells showing hyperchromatic nuclei with eosinophilic cytoplasm and intranuclear inclusions/pseudoinclusions would be key histologic features that are helpful for establishing a diagnosis of GCE. This is particularly true to our case; the presence of giant cells with intranuclear inclusions and papillary structures was a critical clue to intraoperative frozen diagnosis. In addition to the cytologic features, the clinical and radiologic findings are helpful for improving the diagnostic accuracy.
Due to a relatively smaller number of reported cases, we failed to establish the relationship between the histological pattern of GCE and its prognosis. In patients with anaplastic GCE, however, a poor prognosis is expected with a relatively higher rate of recurrence (Table 1). In our patient, there was no disease progression or recurrence. Due to a shorter length of follow-up, however, further long-term follow-up studies are warranted to predict clinical outcomes of anaplastic GCE.
Acknowledgments
This work was supported by the Basic Science Research Program through a National Research Foundation grant funded by Korean Ministry of Education, Science & Technology to Se Hoon Kim (2010-0021092).
Notes
No potential conflict of interest relevant to this article was reported.