Histopathological Causes of Late Liver Allograft Dysfunction: Analysis at a Single Institution
Article information
Abstract
Background
We summarize our experience in the pathological diagnosis of late complications of liver transplantation (LT) to better understand the causes of late allograft dysfunction in a population mostly composed of patients with hepatitis B virus (HBV) infection.
Methods
We reviewed 361 post-transplant liver biopsies from 174 patients who underwent LT and first presented with liver function abnormalities 3 months post-procedure. The underlying diseases included HBV-associated liver disease (77%), toxic or alcoholic liver disease (10.3%), hepatitis C virus (HCV)-associated liver disease (8.6%), primary biliary cirrhosis (1.2%), primary sclerosing cholangitis (1.2%), and metabolic disease (1.7%).
Results
The three most common late complications were acute rejection (32.5%), recurrent disease (19.1%), and biliary complication (17.1%). Patients who underwent LT for HBV infection or for drug- or alcohol-related liver disease had a lower incidence of recurring disease than those who underwent transplantation for HCV infection. During post-transplantation months 3-12, acute rejection was the most common cause of allograft dysfunction and recurring disease was the leading cause for allograft dysfunction (p=0.039). The two primary causes of late allograft dysfunction have overlapping histological features, although acute rejection more frequently showed bile duct damage and vascular endothelialitis than recurring HBV infection, and recurring HBV infection had more frequent lobular activity and piecemeal necrosis.
Conclusions
The causes of late liver allograft dysfunction are closely associated with the original liver diseases and the period after LT. Careful attention is required for differential diagnosis between acute rejection and recurrent HBV.
Liver transplantation (LT) is the standard of care for end-stage, irreversible liver disease. Recently, early mortality and allograft dysfunction rates after LT have fallen dramatically as a result of the development of new surgical techniques, improved preoperative management, and more effective immunosuppressants, thereby increasing the number of patients requiring long-term follow-up.1,2 Therefore, the need to understand the causes of late allograft dysfunction is becoming increasingly more important.
In the initial years after the development of LT procedures, ischemic and reperfusion injury, acute cellular rejection, and the effects of surgical and postoperative complications were very common.3,4 Recently, the causes of graft dysfunction are more variable and overlapping clinical, serological, and histological features make pathological diagnosis difficult.
A few studies that described the causes of late allograft dysfunction and their histological findings have focused mostly on pediatric LT in Western populations, of which the etiological background for LT differs from that of Asian populations. Herein, we summarize our experience in the pathological diagnosis of allograft liver biopsies from LT recipients with late complications to enhance the understanding of the causes and histological findings of late allograft dysfunction.
MATERIALS AND METHODS
Data were obtained from our retrospectively maintained database of orthotopic LT recipients at Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea. A total of 2,112 consecutive patients who underwent liver allograft transplantation between January 1, 2000 and December 31, 2010, were evaluated. The indications for LT were hepatitis B virus (HBV)-associated liver disease (n=1,759), toxic or alcoholic liver disease (n=215), hepatitis C virus (HCV)-associated liver disease (n=84), autoimmune hepatitis (n=7), primary biliary cirrhosis (PBC; n=18), primary sclerosing cholangitis (PSC; n=2), and metabolic disease (n=26). Our standard immunosuppression regimen was either tacrolimus or cyclosporine with steroids.
If allograft liver biopsies were performed more than 3 months post-transplantation because of abnormalities in follow-up liver function test results (elevated alanine aminotransferase and aspartate aminotransferase levels, with or without elevated gamma-glutamyl transferase levels), the case was defined as late allograft dysfunction. Using an electronic database, we selected 174 recipients from the 2,112 LT recipients as the study population. The indications for LT in these 174 patients were HBV-associated liver disease (n=134), toxic or alcoholic liver disease (n=18), HCV-associated liver disease (n=15), PBC (n=2), PSC (n=2), and metabolic disease (n=3). The demographics of the study population are summarized in Table 1.
The total number of liver biopsies was 361 and on average each patient underwent 2 biopsies (range, 1 to 10). If multiple biopsies performed in the same patient in a short period resulted in the same diagnosis, the biopsy results were considered to represent the same episode of late allograft dysfunction and counted as one event. If biopsies were performed over an extended duration of time, each biopsy was counted separately. A total of 245 episodes of late allograft dysfunction were identified including: biopsies of HBV-associated liver disease (n=182), HCV-associated liver disease (n=25), toxic or alcoholic liver disease (n=29), PBC (n=3), PSC (n=2), and metabolic liver disease (n=4).
The causes of late allograft dysfunction were determined by reviewing histological, serological, and radiological findings at the time of late allograft dysfunction and each patient's clinical course after allograft liver biopsy. Transplant recipient data were retrieved from electronic medical records. Data included each patient sex, age at diagnosis of liver disease, treatment, clinical course, and serological test results, including HBsAg, HBcAg, HBV DNA, and HCV RNA. All patients underwent Doppler ultrasound evaluation of the liver, biliary tree, and hepatic vasculature and some underwent computed tomography and endoscopic retrograde cholangiopancreatography. We recorded any evidence of biliary tract dysfunction or blood flow abnormality revealed by these ancillary tests. Biliary complication was defined as a biliary obstruction or stricture proved radiologically or endoscopically. Supporting histologic evidences for biliary complication included portal fibrosis, bile ductular proliferation, neutrophilic or eosinophilic infiltration, as well as centrilobular cholestasis to some degree. Biliary problems caused by other events, such as acute or chronic rejection and recurrent viral hepatitis were not included.
For the purpose of classification and discussion, we have arbitrarily classified liver allograft dysfunction into three time periods after LT: 1) early-late period (ELP) allograft dysfunction (>3 months but ≤6 months after LT); 2) mid-late period (MLP) allograft dysfunction (>6 months but ≤12 months after LT); and 3) late-late period (LLP) allograft dysfunction (>12 months after LT).
Biopsy specimens of allograft livers were fixed in 10% phosphate-buffered formalin and stained with hematoxylin and eosin using the Masson trichrome technique and for cytokeratin 19 (monoclonal mouse anti-human cytokeratin 19 antibody; Dako North America Inc., Carpiteria, CA, USA). If necessary, in situ hybridization study for detection of cytomegalovirus or Epstein-Barr virus was performed. We decided the histologic diagnosis based on variable histological findings including: 1) the distribution, severity, and composition of portal inflammation; 2) the presence and type of interface hepatitis; 3) bile duct inflammation, damage, and bile ductular proliferation; 4) biliary epithelial senescence changes and small bile duct loss; 5) perivenular mononuclear cell inflammation or hepatocyte dropout; 6) lobular findings, including necroinflammatory activities; 7) the pattern of fibrosis; and 8) cholestasis.
Additional comparisons of histological features were performed between the three primary causes of late allograft dysfunction: acute cellular rejection, recurrent HBV and biliary complication. The compared histological features included duct damage, lobular activity, endothelialitis, piecemeal necrosis, fibrosis, cholestasis, bile ductular proliferation, and composition of inflammatory cells. Two pathologists blinded to patients' clinical histories reviewed all slides. After interpreting the histological findings, conflicting findings were discussed with both pathologists. If the pathologic finding was not conclusive or discrepant with the clinical finding, final diagnosis was determined based on clinical findings and the diagnosis remained indeterminate, when decision of final diagnosis was difficult in spite of all effort. Groups were compared using Pearson's χ2 and student t-tests. The results were considered statistically significant at p<0.05. All statistical analyses were performed using SPSS ver. 12.0 (SPSS Inc., Chicago, IL, USA).
RESULTS
Frequencies of post-transplantation hepatic dysfunction
At least one episode of allograft dysfunction was diagnosed in 819 (38.78%) of the 2,112 patients. Among them, 645 (78.75%) had early allograft dysfunction and 174 (21.25%) had late allograft dysfunction. The frequencies of overall allograft dysfunction were significantly different according to the underlying liver disease (p<0.001). Recipients with HCV infections showed allograft dysfunction most frequently (75%), whereas the recipients who had alcohol- or drug-associated liver disease displayed the lowest rates of allograft dysfunction (35.81%). However, rates of late allograft dysfunction were not different between groups and ranged from 15.31% to 23.52% in overall allograft dysfunction (Fig. 1).
Causes of late allograft dysfunction
The three most common late complications were acute rejection (32.5%), recurrent disease (19.1%), and biliary complication (17.1%). In 22 cases (8.9%), the histological changes were minimal and nonspecific, with minimal portal or lobular inflammation. In these cases functional, radiological and serological testing showed no abnormalities, thus the cause of late allograft dysfunction could not be determined. In most of these cases, the clinical course was good and patients recovered without immunosuppressant or antiviral treatment, or radiological intervention. In 22 cases (8.9%), combined causes contributed to late allograft dysfunction, among which combined acute cellular rejection and biliary complication were the most frequent combinations (9/22, 40.9%) (Table 2).
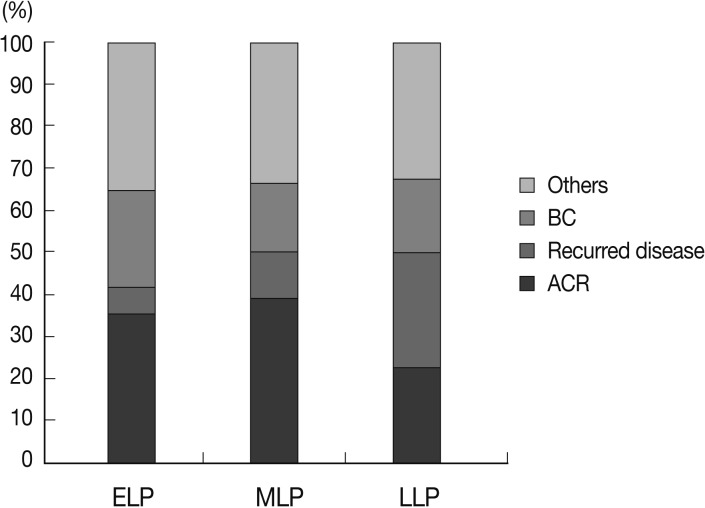
In the recipients with HBV infections, acute cellular rejection was the most frequent cause of late allograft dysfunction (53/182, 29.1%) and recurrent HBV hepatitis and bile duct damage contributed equally to late allograft dysfunction (each, 34/182, 18.7%). In patients with HCV infections, recurrent hepatitis was the major cause of late allograft dysfunction (10/25, 40%). In the cases of toxic or alcoholic liver disease, acute cellular rejection (14/29, 48.3%) contributed greatly to late allograft dysfunction with a low frequency of recurrent disease (2/29, 6.9%). The intergroup differences were statistically significant (p=0.048). Fig. 2 summarizes the causes of late allograft dysfunction according to patients' underlying liver diseases.
Causes of late allograft dysfunction according to underlying liver diseases before liver transplantation. HBV, hepatitis B virus; HCV, hepatitis C virus; BC, biliary complication; ACR, acute cellular rejection.
Two of three patients with PSC demonstrated acute cellular rejection, and one showed recurrent disease 4 years after LT. A patient with PBC underwent retransplantation because of chronic rejection 5 years and 3 months post-LT and presented with acute cellular rejection 4 months after retransplantation. Acute cellular rejection occurred in the two recipients with Wilson disease and in one with glycogen storage disease. One recipient with Wilson disease had biliary tract stricture.
Eight of 13 chronic rejection cases were preceded by acute cellular rejection.
Causes of late allograft dysfunction by follow-up period in patients with HBV infection
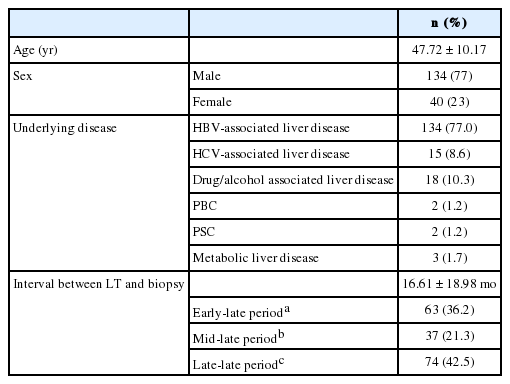
We analyzed the causes of late allograft dysfunction according to the follow-up period, including ELP (n=48, 26.4%), MLP (n=36, 19.8%), and LLP (n=98, 53.8%). There was a significant difference in the causes of allograft dysfunction between groups (p=0.039).
In ELP and MLP, acute cellular rejection was the most common cause of allograft dysfunction (17/48, 35.4% and 14/36, 38.9%, respectively). In LLP, however, the frequency of acute cellular rejection was significantly lower (22/98, 22.4%). In LLP, recurrent disease was the most frequent cause of allograft dysfunction (27/98, 27.6%). Biliary complication caused allograft dysfunction more frequently in ELP (11/48, 22.9%) than in MLP (6/36, 16.7%) or LLP (17/98, 17.3%) (Fig. 3).
Comparison of histological features of acute cellular rejection, recurrent disease and biliary complication in recipients with HBV infection
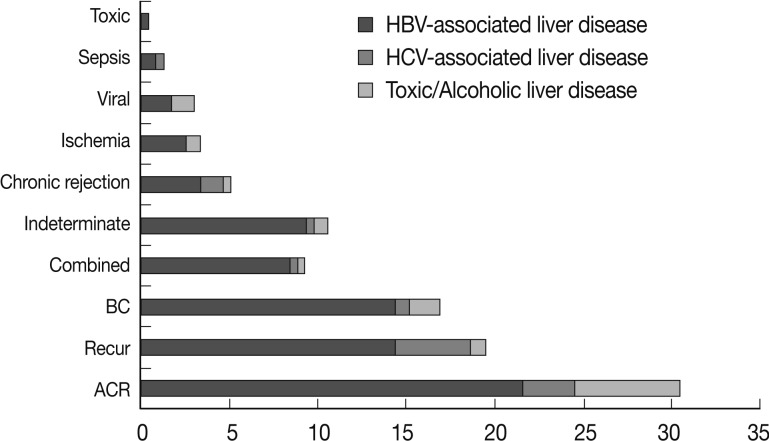
Several histological parameters were compared between acute cellular rejection, recurrent HBV hepatitis and biliary complication occurring in the late post-transplantation period (>3 months post-transplantation). Classical histological features of acute cellular rejection, such as bile duct damage or vascular endothelialitis were more frequently observed in acute cellular rejection than in recurrent HBV-associated hepatitis or biliary complication (p<0.001 and p=0.001, respectively), but their incidence rates were also comparatively higher in recurrent HBV-associated hepatitis (60% and 35.41%, respectively). The frequencies of lobular activity were significantly lower in biliary complications with no differences between the two other groups (p=0.022). Piecemeal necrosis was more commonly observed in recurrent HBV-associated hepatitis (p=0.011). Fibrosis, cholestasis and bile ductular proliferation showed no statistical differences between groups, although fibrosis was quite frequent in recurrent HBV-associated hepatitis and cholestasis was frequent in biliary complication. The presence of neutrophils in the infiltrating inflammatory cell population was significantly more frequent in biliary complications (p<0.001). Fig. 4 summarizes the histological comparison between late acute rejection (LAR), recurrent HBV-associated hepatitis and biliary complications. Fig. 5 shows the representative histological features of these three primary causes of late allograft dysfunction.
Histological comparison between late acute rejection and recurrent hepatitis B virus (HBV)-associated hepatitis. ACR, acute cellular rejection; PMN, piecemeal necrosis.
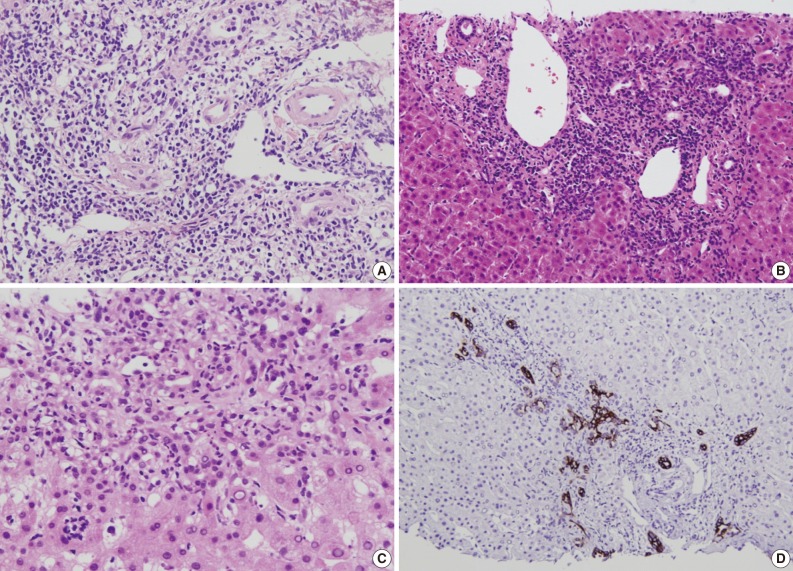
Representative features of major causes of late allograft dysfunction. (A) Acute cellular rejection: bile duct damage and vascular endothelialitis. (B) Recurrent hepatitis B virus-associated hepatitis: lymphoplasma cells infiltration in portal tract with interface activity. Biliary complication; neutrophilic infiltration in portal tract (C) and bile ductular proliferation (cytokeratin 19) (D).
DISCUSSION
The spectrum of pathological findings encountered in liver allografts is broad, encompassing immunological processes, viral infections, drugs, ischemia, and recurrent disease.5 Particularly in the late post-transplantation period, the causes of allograft dysfunction are more variable and complicated, often making diagnosis quite difficult.
The definition for "late" allograft dysfunction has not been established. In previous studies, a period longer than 6 months was frequently used to define late allograft dysfunction.3,5,6 The first 2 to 3 months, in which acute cellular rejection is relatively common, was sometimes considered the early allograft period, and the period between 3 to 6 months was an intermediate allograft period.6,7 We considered a period longer than 3 months post-LT as the late allograft period and used arbitrary classifications of late allograft dysfunction by follow-up period. Using this classification, we found that the etiology of hepatic allograft dysfunction dramatically changed 1 year post-LT. Acute cellular rejection was the leading cause of allograft dysfunction in ELP and MLP, but recurrent disease was the most common cause of allograft dysfunction in LLP. Biliary complication decreased slightly in frequency in LLP. Pappo et al.8 described the causes of allograft dysfunction occurring more than 5 years after transplantation, and they reported that viral infection and minimal histological changes of unknown nature were the most frequent findings, followed by rejection, recurrence of the original disease, and obstructive cholangiopathy. These findings demonstrate that the causes of allograft dysfunction are dependent upon the elapsed time after transplantation.
In the present study, 38.8% of the recipients underwent liver biopsies because of allograft dysfunction all of which occurred within the first 3 months (78.75%). After 3 months, the rate of allograft dysfunction declined (21.25%). Recurrence of the recipient's original liver disease was the most common cause of late allograft dysfunction in previous reports.5,7 However, acute cellular rejection was the leading cause of allograft dysfunction until 1 year after LT in our study population, which was predominantly comprised of HBV-associated liver disease cases. The rate of recurrence of HBV fell dramatically because of the introduction of human HBV immunoglobulins and nucleoside analogs.9 Although some antiviral agents, such as ribavirin or interferon, have been used as prophylactic therapy to prevent HCV infection in liver grafts, it is not clear whether these agents are of any benefit to patients.10,11 The response to antiviral therapies may contribute to the causal difference in late allograft dysfunction between the HBV and HCV.
Idiopathic post-transplantation hepatitis (IPTH) in an allograft liver is defined as the presence of a portal and lobular mononuclear infiltrate in the absence of evidence of rejection and other identifiable causes of graft damage.5,12 In the present study, 22 cases (8.9%) showing minimal portal or lobular inflammation with no functional, or radiological abnormalities may be assumed to be IPTH. The prevalence of IPTH is difficult to determine, and its incidence rate ranges from <10% to approximately 50% in different series.6 In a population comprising patients who underwent protocol biopsy, IPTH was frequent, whereas IPTH was relatively rare when biopsies were performed on the basis of abnormal liver function test results.12-14 The prognosis for IPTH is not yet clear, however the degree of fibrosis is aggravated in some cases and often progresses to cirrhosis.15,16 Unfortunately, this study's long-term follow-up biopsies of IPTH cases were not available, although the short-term clinical courses of these patients were relatively good.
The incidence of chronic rejection in the present study was less than 1%, lower than that of a previous report (<3-4%). The declining incidence of chronic rejection may be due to improved management of acute rejection with immunosuppression and the widespread use of liver biopsy procedures.17,18
The definition of "LAR" is variable. Some define LAR as acute rejection occurring later than 3 months post-LT, while others consider acute rejection occurring later than 6 months.6,7,19,20 In the present study, an acute rejection occurring 3 months post-LT was diagnosed as LAR in 81 biopsies from 76 (3.6%) of 2,112 recipients. In previous studies, the incidence rates of LAR have been reported at 7-18%.2,19,21 Higher frequencies may be related to stricter biopsy protocols and longer follow-up periods. LAR is known to display a different pattern and nature of inflammatory infiltrates than early acute rejection, including more frequent interface and lobular activity.6 We compared the histological features of LAR and recurrent HBV infection occurring later than 3 months post-LT. The hallmarks of viral hepatitis, including piecemeal necrosis and fibrosis, were less frequently observed in LAR. Bile duct damage and perivenular endothelialitis were more frequently found in LAR. However, these findings were not infrequently observed in recurrent HBV hepatitis. The frequency of lobular activity was similar, leading to difficulty in differential diagnosis. Because of these overlapping features, it is important to correlate biopsy findings with liver function test results, serological tests for viruses, and a review of previous biopsies to determine a differential diagnosis between LAR and recurrent HBV liver disease.22 As for biliary complications, the presence of neutrophils was significant, unlike acute rejection and recurrent HBV-associated hepatitis, but the frequencies of the other histologic features of biliary complications including cholestasis or bile ductular proliferation showed no between-group differences.
In conclusion, the causes of late liver allograft dysfunction are closely associated with the original liver disease and the period after LT. Overlapping histological features of LAR and recurrent HBV hepatitis make definitive diagnosis more difficult and require more careful attention.
Notes
No potential conflict of interest relevant to this article was reported.