Acinar Cell Carcinoma of the Pancreas: Clinical and Cytomorphologic Characteristics
Article information
Abstract
Acinar cell carcinoma is a rare malignant epithelial neoplasm with predominantly exocrine acinar differentiation and is seen primarily in older men (mean age, 62 years). The presenting symptoms are usually non-specific, and jaundice is often not present. Symptoms relating to the overproduction and release of lipase into the circulation are present in 10-15% of patients. Characteristic cytomorphologic features include a population of cells with minimal pleomorphism, eccentrically placed nuclei with a single prominent nucleoli and moderate hyperchromasia. The cytoplasm is finely granular, and the background may contain granular debris secondary to cytolysis. A significant proportion of the cases also have a minor neuroendocrine component or scattered neuroendocrine cells. Approximately 50% of patients have metastatic disease at presentation, often restricted to the regional lymph nodes and liver. The prognosis is poor, only slightly better than that of pancreatic ductal adenocarcinoma.
CLINICO-RADIOLOGIC CHARACTERIZATION
Acinar differentiation within the exocrine pancreas is characterized by the expression of pancreatic enzymes including trypsin, chymotrypsin, and lipase stored within large (125-1,000 nm) cytoplasmic zymogen granules.1-3 Acinar neoplasms are almost all malignant, except for the extremely uncommon acinar cystadenoma.4-6 Acinar cell carcinoma (ACC) is a rare pancreatic neoplasm accounting for approximately 1% of carcinomas of the exocrine pancreas in adults and 15% in the pediatric population. 1
ACC patients show a male predominance (3:1)7 and a bimodal age distribution with one peak in childhood and a second in older adults.8,9 ACC may arise anywhere in the pancreas and averages 10 cm in size at the time of diagnosis.10,11 The tumor has also reportedly arisen outside the pancreas as a result of pancreatic acinar heterotopia/metaplasia.12-15
Presenting symptoms and imaging studies are generally nonspecific, except for a subset of patients (10-15%) with symptoms relating to lipase hypersecretion who develop polyarthralgia and painful subcutaneous fat necrosis/nodules.2,16-19 In the absence of a known pancreatic mass, erythema nodosum is often initially considered the most likely diagnosis in these patients.19-21 Although elevated lipase has been identified in half of ACC patients, only a small percentage of patients with levels greater than 1,000 U/dL will develop symptoms of lipase hypersecretion.22 Several studies have also reported elevated serum alphafetoprotein (AFP) levels in patients with metastatic ACC.1,3,23,24 In these patients, the serum AFP level can be used to monitor the effectiveness of treatment. Other more common tumor markers such as carcinoembryonic antigen and cancer antigen 19-9 are generally not elevated.25
ACC is considered a high-grade malignancy, with 50% of patients presenting with metastatic disease, while an additional 25% with organ-confined disease later manifest either regional or distant recurrence.26 ACC is more often limited to the pancreas at the time of diagnosis relative to pancreatic ductal adenocarcinoma (PDA), attributed to ACC showing lower rates of vascular invasion and lymph node involvement.25 Meta-analysis of multiple case series found overall five-year survival rates ranging from 36-72%.8,9,25 Studies following patients with surgically resectable pancreatic disease have shown significantly better survival, greater than 40% at five years.25
Surgery offers the best chance for long-term survival. Large studies examining the prognosis of ACC reported a median survival of 47 months in general and 123 months in patients with surgically resectable disease.8,27 Notably, while patients with metastatic PDA are generally not considered surgical candidates, over 25% of patients with known metastatic ACC undergo surgical resection of both the primary and metastatic lesions. This is due largely to the uncertain long-term outcome and known resistance of ACC to standard chemoradiation regimens. 25,28
Most ACCs are large, easily appreciated on radiological examination, and tend to have more clearly defined borders than PDAs. Imaging may show variable fluorodeoxyglucose uptake in areas that appear heterogeneous with contrast enhancement on computed tomography.17 Internal calcifications and central hypodense regions are also common. The presence of internal hemorrhage, however, is unusual in ACC and increases the possibility of a solid-pseudopapillary neoplasm (SPN).17
Molecular and cytogenetics
The ACC molecular features differ significantly from those of PDA, as ACC typically do not have KRAS, TP53, and SMAD4 mutations.29,30 In contrast, approximately 25-50% of ACCs show a loss of heterozygosity on chromosome 11p and have mutations in the APC-β-catenin pathway, which is commonly mutated in colorectal carcinoma.30,31 Interestingly, the rationale for evaluating chromosome 11p arose from the observation that a subset of patients predisposed to pancreatoblastoma have Beckwith-Wiedemann syndrome. Pancreatoblastoma shares morphological and immunohistochemical features with ACC, as well as several genetic similarities.31 Mutations in the APC-β-catenin pathway, whether germline or sporadic, create a markedly increased risk for colonic and extracolonic carcinomas. The importance of identifying genetic alterations in the APC-β-catenin pathway provides a theoretical potential use for chemotherapeutics such as 5-fluorouracil, irinotecan, and oxaliplatin which have shown a significant survival benefit in patients with advanced colorectal carcinoma. However, such an approach must be established in clinical trials.32,33 MicroRNA studies have identified overexpression of miR-17, miR-20, and miR-92-1 in cultured cells from pancreatic tumors.34 miR-92-1 was previously found to act as an oncogene with the MYC gene, and mouse models with MYC induction of acinar cells produced acinar/ductal adenocarcinomas.35
MORPHOLOGICAL CHARACTERISTICS
Histopathology of ACC
ACC grossly appears relatively well-circumscribed with a tan to red soft cut surface and occasionally central necrosis. The soft texture reflects a paucity of fibrous stroma within the tumor (Fig. 1). Microscopically, well-differentiated ACCs usually have prominent acinar formations without lobular arrangements. These acinar arrangements may be confused with the "rosettes" of a pancreatic neuroendocrine tumor (Figs. 2, 3). A characteristic feature and clue to the diagnosis is the presence of single prominent nucleoli. Other cell components (i.e., ductal and endocrine cells) are often absent. ACC with moderate differentiation lacks the compact, lobular arrangement of benign acinar epithelium and are clustered more haphazardly. Poorly-differentiated ACC can form sheets of cells without well-defined acinar structures.
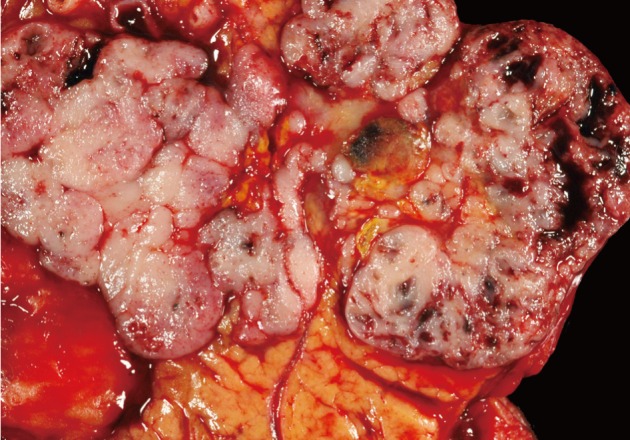
Acinar cell carcinoma (ACC). ACCs are typically large (10 cm), fairly well-circumscribed, soft and fleshy. Less commonly, they can be fibrotic or form cystic masses. Other pancreatic neoplasms that form fleshy masses include well-differentiated pancreatic neuroendocrine tumor, pancreatoblastoma, solid-pseudopapillary neoplasm, and lymphoma.
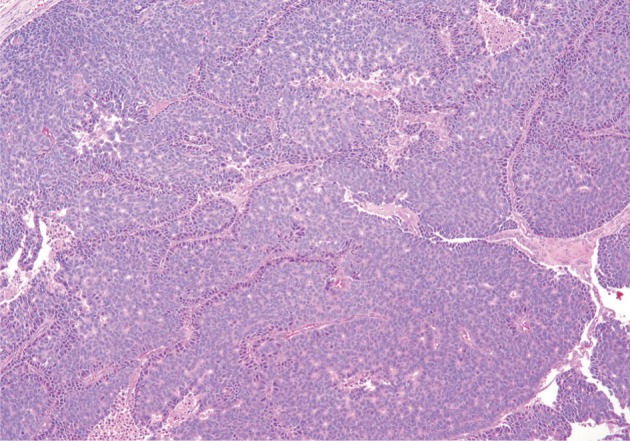
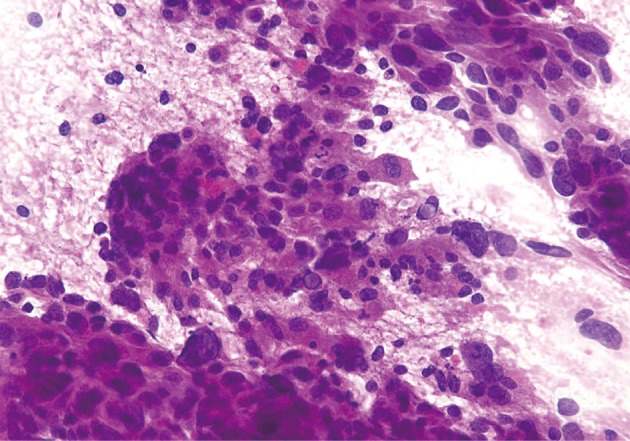
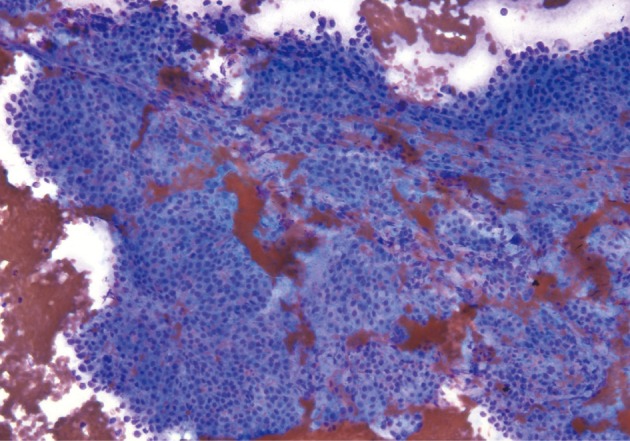
Acinar cell carcinoma. Neoplastic cells show acinar formations, relative cellular monotony, and foci of necrosis. Thin bands of fibrosis separate islands of neoplastic cells.
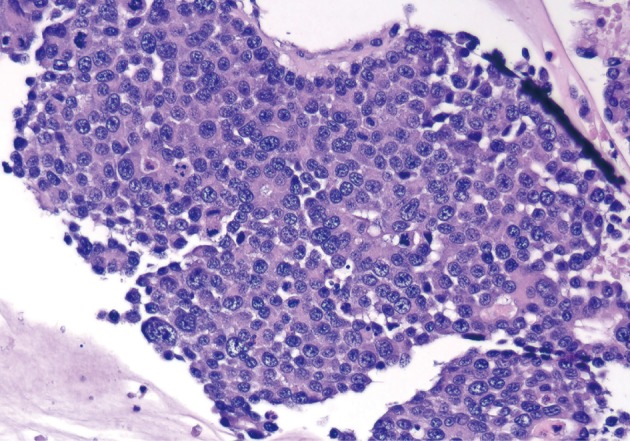
Acinar cell carcinoma. Poorly differentiated neoplastic cells with focal acinar formations, large nuclei exhibiting irregular chromatin clumping, size variation, and varying N/C ratios. Many mitotic figures are present, consistent with aggressive growth.
Immunohistochemical labeling for trypsin, lipase, and chymotrypsin can be used to demonstrate the production of pancreatic exocrine enzymes by the neoplastic cells. The neoplastic cells also usually express cytokeratin (keratin 8 and 18, CAM5.2, AE1/AE3). Immunolabeling for chromogranin or synaptophysin will demonstrate a minor neuroendocrine component in one-third of ACC cases. When more than 30% of the neoplastic cells are labeled with the neuroendocrine markers, the diagnosis of a mixed acinar-neuroendocrine carcinoma should be considered. Apical intracytoplasmic granules can be highlighted by a periodic acid-Schiff stain after diastase digestion (PAS-D).
ACC cytopathology
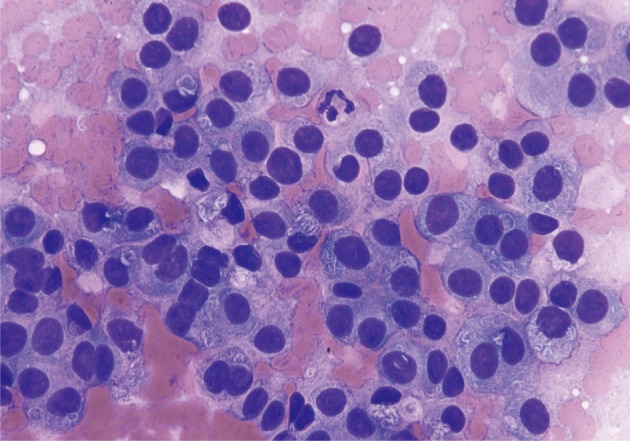
ACC smears are usually hypercellular, mostly small- to midsized cohesive cellular fragments with few single cells. Often, the cytoplasm appears clear and fragile, resembling acinic cell carcinoma of the parotid gland, and resulting in a population of naked nuclei resembling lymphocytes in the smear background (Figs. 4-6). A densely granular background may also be present. The overall impression should be a population of cells larger than PDA, moderately hyperchromatic with minimal pleomorphism and eccentrically- or basally-located nuclei. Nuclear features include coarse chromatin, nuclear pseudoinclusions, and single prominent nucleoli. There is finely granular or vacuolated cytoplasm which often appears basophilic and usually appears metachromatic on staining. The characteristic cytological features of ACC including acinar formations are less apparent as the tumor becomes more severe, with bizarre, pleomorphic nuclei and macronucleoli (Figs. 7, 8).
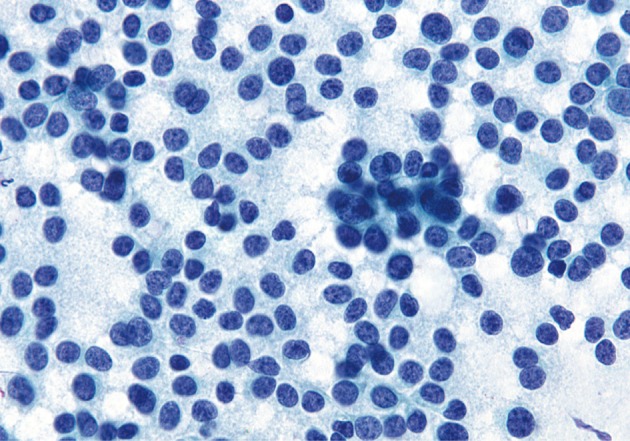
Acinar cell carcinoma. Partially intact tissue fragment with neoplastic cells has moderately hyperchromatic, uniform nuclei and delicate basophilic cytoplasm. A lack of single dispersed cells may help to differentiate this from a pancreatic neuroendocrine tumor (Diff-Quik stain).
Acinar cell carcinoma. Neoplastic cells arranged singly or in groups. There are round to ovoid nuclei with uniform chromatin patterns and delicate, fragile cytoplasm. While these features are typical of acinar cell carcinomas, they may also be present in some well-differentiated pancreatic neuroendocrine tumors (Papanicolaou stain).
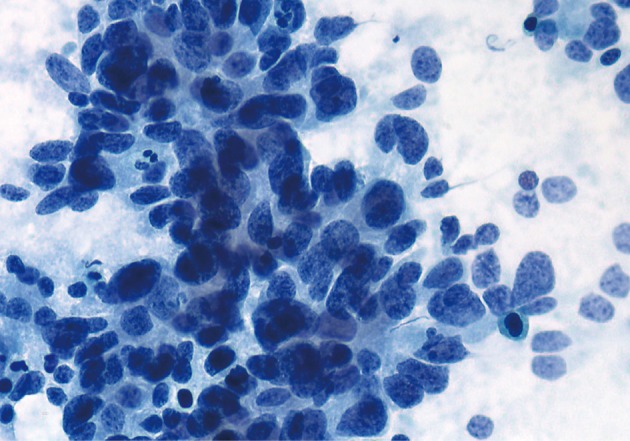
Acinar cell carcinoma (ACC). Poorly differentiated ACC. Neoplastic cells are disorganized, but several atypical acinar formations are present. The acinar formations can be easily confused with "rosettes" of an endocrine neoplasm (Papanicolaou stain).
DIFFERENTIAL DIAGNOSIS
Pancreatic neuroendocrine tumor
Neuroendocrine tumors can mimic ACC both cytologically and histologically. Both neoplasms can have abundant eosinophilic cytoplasm with an acinar arrangement. Features favoring ACC include prominent nucleoli, granular eosinophilic cytoplasm, and minimal fibrotic stroma. Both neoplasms can also have overlapping immunolabeling patterns. Approximately 30% of ACC cases will label for the neuroendocrine markers chromogranin and synaptophysin, and neuroendocrine tumors may focally express acinar markers.36,37 ACC should demonstrate diffuse positive labeling for multiple acinar markers (e.g., trypsin, chymotrypsin).
Smears of neuroendocrine tumors are moderately to highly cellular; scant cellularity is common with cystic degeneration. There will be an abundance of monotonous small- to medium-sized polygonal cells with or without small- to medium-sized groups or rosettes. Although nuclei are usually bland with coarse or stippled chromatin, there can be marked atypia with significant pleomorphism, so-called endocrine atypia that does not carry clinical significance. Nucleoli are usually inconspicuous but occasionally quite prominent. Cytoplasm is relatively scant and usually dense and eccentric, yielding a plasmacytoid appearance (best appreciated in single cells). Rare variants produce cells with clear, vacuolated, or oncocytic cytoplasm. Mitotic figures and necrosis are absent to rare. A vascular network or bloody background may or may not be present.
Pancreatoblastoma
Pancreatoblastoma is the only other pancreatic malignancy with extensive acinar differentiation. Pancreatoblastoma and ACC have similar cytopathological and genetic features, and pancreatoblastoma is regarded by some as the pediatric variant of ACC generally occurring before ten years of age.1,3,7,38 The only microscopic difference between the lesions is the presence of squamoid nests in pancreatoblastoma.
Smears of pancreatoblastoma are hypercellular with tissue fragments of varying sizes arranged in solid sheets, three-dimensional loosely cohesive epithelial groups, and abundant stromal tissue. The epithelial component forms acini, nests, and organoid patterns. Cells in the acinar formations have a slightly elongated columnar shape with more abundant delicate cytoplasm and basally-placed nuclei and indistinct nucleoli. Cells may also be spindle-shaped, elongated, or triangular in pattern. Focally, the large epithelial cells form swirling eddies consistent with squamoid corpuscles, often best appreciated on cell block. Primitive spindled mesenchymal tissue may also be present, rarely showing cartilage formation.
Pancreatic ductal adenocarcinoma
In cases of poorly-differentiated ACC, the differential includes PDA. Features of PDA are similar to those of other upper gastrointestinal or pancreatobiliary adenocarcinomas. Mucin production is a specific sign for ductal differentiation. Nuclei show marked nuclear pleomorphism and nucleoli with loss of nuclear polarity. Most tumors show moderate to poor differentiation with abortive tubular structures and abundant mitoses (>10/10 high power fields). Well-differentiated PDA may appear as benign ducts but with an irregular shape and distribution. The basal cytoplasm may show mucinous vacuoles.
Solid-pseudopapillary neoplasm
The last tumor which may mimic ACC is a SPN. These may also be composed of uniform polygonal cells similar in appearance to ACC. In contrast, SPN should form acini and may often have prominent degenerative features (responsible for the pseudopapillary appearance). Immunohistochemistry of SPN shows expression of alpha-1-antitrypsin, nuclear β-catenin, and CD10.1 CD56 is another non-specific marker which may be positive in both tumors.
Smears of SPN are composed of small, uniform cells in cohesive, often branching and papillary cell clusters. Individual cells are homogeneous in appearance with weak anisonucleosis. Nuclei are round to oval with inconspicuous nucleoli, smooth to slightly indented or grooved nuclear membranes, and finely granular chromatin. Mitotic activity is usually not seen. Cytoplasm is scant to moderate, non-granular to finely granular, and may contain a small perinuclear vacuole or intracytoplasmic hyaline globule. Delicate fibrovascular cores are often present with myxoid stroma (Romanowsky stain shows magenta colored and metachromatic material; PAS positive, diastase resistant). A zone of cytoplasm often separates the nuclei of the neoplastic cells from the fibrovascular cores. The background may be clean or filled with hemorrhagic cyst debris laden with foamy histiocytes and multinucleated giant cells.
Carcinomas with mixed differentiation
Pancreatic neoplasms may occasionally show multiple lines of differentiation including acinar-endocrine, acinar-ductal, and acinar-endocrine-ductal.37-40 Regardless of tumor composition, they are all clinically aggressive. The diagnostic criteria for a mixed tumor require each component to comprise a minimum of 30% of the lesion. The most common and best characterized is the acinar-neuroendocrine carcinoma. Due to considerable cytomorphologic overlap, accurate quantification of the cell lineages is only possible through immunohistochemical studies.
FUTURE DIRECTIONS
Surgery currently remains the mainstay of curative treatment. Patients with non-operable or metastatic disease have few options, as there is no consensus on medical therapy in this patient population.11 Small case series and reports have attempted a variety of regimens including single or multidrug chemotherapy, as well as chemoradiation.41 Case series have reported partial response in patients receiving 5-fluorouracil-based regimens.41 Responses were also seen in patients treated with S-1, a type of fluoropyrimidine. Metastatic ACC lesions are characteristically hypervascular, making newer drugs targeting vascular proliferation another potential opportunity to improve outcome.11 Our ongoing research using next-generation sequencing identified multiple hotspot mutations in BRAF, a known potential drug target (unpublished data). Due to the rarity of ACC, multicenter and multinational cooperative trials are necessary to establish standardized treatment protocols.
Notes
No potential conflict of interest relevant to this article was reported.