No Detection of Simian Virus 40 in Malignant Mesothelioma in Korea
Article information
Abstract
Background
Simian virus 40 (SV40), a polyomavirus, was discovered as a contaminant of a human polio vaccine in the 1960s. It is known that malignant mesothelioma (MM) is associated with SV40, and that the virus works as a cofactor to the carcinogenetic effects of asbestos. However, the reports about the correlation between SV40 and MM have not been consistent. The purpose of this study is to identify SV40 in MM tissue in Korea through detection of SV40 protein and DNA.
Methods
We analyzed 62 cases of available paraffin-blocks enrolled through the Korean Malignant Mesothelioma Surveillance System and performed immunohistochemistry for SV40 protein and real-time polymerase chain reaction (PCR) for SV40 DNA.
Results
Of 62 total cases, 40 had disease involving the pleura (64.5%), and 29 (46.8%) were found to be of the epithelioid subtype. Immunostaining demonstrated that all examined tissues were negative for SV40 protein. Sufficient DNA was extracted for real-time PCR analysis from 36 cases. Quantitative PCR of these samples showed no increase in SV40 transcript compared to the negative controls.
Conclusions
SV40 is not associated with the development of MM in Korea.
Simian virus 40 (SV40) is a polyomavirus that originates from the rhesus macaque and was discovered in 1960 as a contaminant of human polio vaccines produced in monkey cells.1 Recently, there has been considerable interest in the identification of SV40 in human tumors, including malignant mesothelioma (MM) and other rare tumors such as osteosarcoma, ependymoma, and choroid plexus tumors.2-6 Subsequently, it has been found that SV40 may play an important role in the etiology of these tumors.2-6 However, SV40 was not detected in malignant lymphomas in Korean patients.7 Oncogenesis driven by SV40 is mediated by the large tumor antigen (T-Ag) oncoprotein, which is capable of transforming different types of cells in the absence of other viral genes.8,9 T-Ag induces DNA synthesis in host cells and prolongs the onset of the S-phase through the inhibition of tumor suppressor proteins p53 and Rb.8
MM is a rare and extremely aggressive neoplasm that arises from the serosal surfaces of pleural, peritoneal, and pericardial cavities and is very likely associated with amphibole asbestos exposure.10,11 In recent years, the incidence of MM has been gradually increasing in concert with industrial development and increased exposure to asbestos.12 However, only a small number of people who have been exposed to asbestos during their lifetime will develop MM.11 This suggests that SV40 may act as an independent carcinogenic agent or as a cofactor of asbestos to augment the risk of MM.13 Studies investigating the relation of SV40 to MM have been inconsistent and differ by geographical location.6
The incidence of MM varies according to geographic location, and the highest incidence rates are reported from Australia, Belgium, and Great Britain.14 In Korea, the incidence of MM is very low compared with other developed countries, with only about one case per million in the last decade.14,15 However, the incidence is recently increasing. Korea has a higher proportion of females (33.8%) among MM cases compared with other nations such as Canada (15.4%) or Italy (27.6%), and most of the patients are diagnosed when they are in their 50s, which is younger compared to cases in other countries.15 Although the majority of MM patients have had occupational (36.8%) and/or environmental (20.4%) asbestos exposure, the remaining patients (42.8%) had no known asbestos exposure.15
The role of SV40 in the oncogenesis of MM following asbestos exposure has not yet been studied in a Korean population. The purpose of this study is to identify SV40 DNA and protein in tissues extracted from MM from a Korean population using quantitative polymerase chain reaction (PCR) and immunohistochemical staining.
MATERIALS AND METHODS
Study population
Using the Korean Malignant Mesothelioma Surveillance (KMMS) tissue bank, 356 cases of MM that occurred between the years 2001 and 2009 were available. Of these, 62 formalin-fixed and paraffin-embedded tissue tumor blocks were selected. The selection was based on the availability of adequate neoplastic tissue within the paraffin blocks. Original diagnoses were based on morphological evaluation in the primary centers and confirmed by three expert pathologists. All cases were analyzed using an immunohistochemical panel (positive marker: calretinin and/or D2-40, WT-1; negative marker: carcinoembryonic antigen). Each tumor slide was re-evaluated to verify the original diagnosis.
Immunohistochemistry
Detection of SV40 protein was performed by an automatic staining procedure using the Ventana Benchmark XT (Roche Diagnostics, Basel, Switzerland). The sections were deparaffinized, then pretreated with CC1 (Roche Diagnostics) for 60 minutes at 42℃. The sections were then washed with reaction buffer and incubated with anti-SV40 antibody (Cell Marque Corp., Rocklin, CA, USA) at a 1:100 dilution for 60 minutes at 42℃. Antibody detection was performed using the UltraView Universal DAB kit (Roche Diagnostics) according to the manufacturer's recommendations. Finally, the slides were counterstained with hematoxylin (Roche Diagnostics) and mounted. As a positive control for SV40 sections from paraffin-embedded tissue, kidney tissue confirmed to contain polyomavirus was used (kindly provided by Professor Young-Mee Cho from the Department of Pathology, Asan Medical Center, Seoul, Korea). The negative controls were performed without the primary antibody.
DNA extraction from paraffin-embedded tissues
The paraffin-embedded tissue blocks were de-paraffinized using xylene. Briefly, the paraffin was removed by two extractions with 1 mL of xylene for 30 minutes and two with 0.5 mL of ethanol and subsequently dried at 37℃. DNA was extracted using the DNeasy Blood and Tissue Kit (Qiagen, Valencia, CA, USA) according to the manufacturer's instructions. Of the paraffin-embedded tissues analyzed, sufficient DNA was only extracted from 36 samples.
β-actin PCR
To ensure the quality of the extracted DNA, all samples were analyzed for the presence of the β-actin gene. DNA (200 ng) was amplified using a HotStar Taq Plus master mix kit (Qiagen). After a denaturing step of 15 minutes at 95℃, 30 amplification cycles were performed. Each cycle consisted of 1 minute at 94℃, 30 seconds at 56℃, and 1 minute at 72℃, followed by a final extension step of 5 minutes at 72℃ using β-actin (forward 5'-CCT TCC TGG GAC TGG AGT CCT-3' and reverse 5'-GGA GCA ATG ATC TTG ATC TTC-3') primers.
SV40 quantitative PCR
A real-time PCR method (TaqMan) for SV40 was previously established which uses a primer pair and an oligonucleotide probe with the fluorescein reporter dye FAM attached to the 5' end and a rhodamine dye (TAMRA) quencher linked to the 3' end. A threshold cycle value (Ct) was calculated for each sample by determining the point at which the fluorescence exceeded the threshold limit chosen for the specific plate.
The real-time PCR assay used the forward primer 5'-CAC AGC ATG ACT CAA AAA ACT TAG CA-3' and reverse primer 5'-GAC TCT CAA CAT TCT ACT CCT CCA AAA-3' and the fluorogenic TaqMan probe 5'-ACC CCA AGG ACT TTC-3'.
Negative controls (water) and positive controls (DNA from the HEK293T cell line) were included on each plate, and a standard curve (SV40 standard solutions) was produced from which the number of genomes in the samples could be calculated. The SV40 quantities used in the standards were 100,000, 10,000, 1,000, 100, and 10 copies per 1 µL (i.e., per well). Thermal cycling, fluorescence detection, and data analysis were performed on an ABI PRISM 7900 Sequence Detector (Applied Biosystems, Foster City, CA, USA) using the software provided by the manufacturer.
The standard stock solution, plasmid pDRIVE, containing the SV40 genome was cultivated in medium, and the culture was purified according to the manufacturer's instructions (Qiagen). The concentration of the plasmid solution was calculated using the absorbance at 260 nm.
Ethics statement
This study was approved by the Institutional Ethics Committee of Yonsei University, Wonju College of Medicine, Wonju, South Korea (IEC No. YWMR-12-4-033) and was in compliance with the Helsinki Declaration.
RESULTS
Clinicopathological characteristics
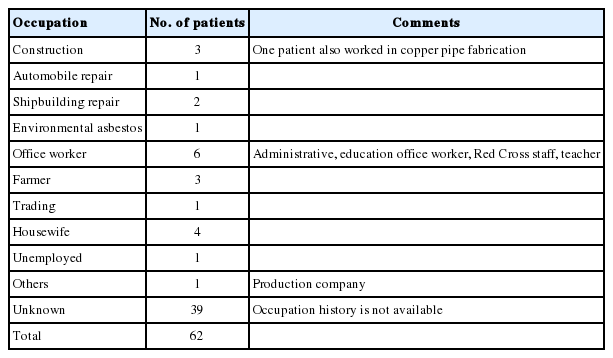
The study included 38 (61.3%) males and 24 (38.7%) females between the ages of 34 and 79 years, with a mean age of 60.2 years. The majority of patients developed MM when they were in their 50s, followed by patients over 70, patients in their 60s, 40s and finally in their 30s (Fig. 1). Most cases of MM occurred in the pleura (40 cases, 64.5%), followed by the peritoneum (21 cases, 33.9%) and the pericardium (one case, 1.6%). With regard to histologic classification, the epithelioid type was the most common histologic type (29 cases, 46.8%), followed by the biphasic (nine cases, 14.5%), sarcomatoid (six cases, 9.7%), desmoplastic types (four cases, 6.5%), and other undetermined variants (14 cases, 22.6%). The clinicopathological data are summarized in Table 1.
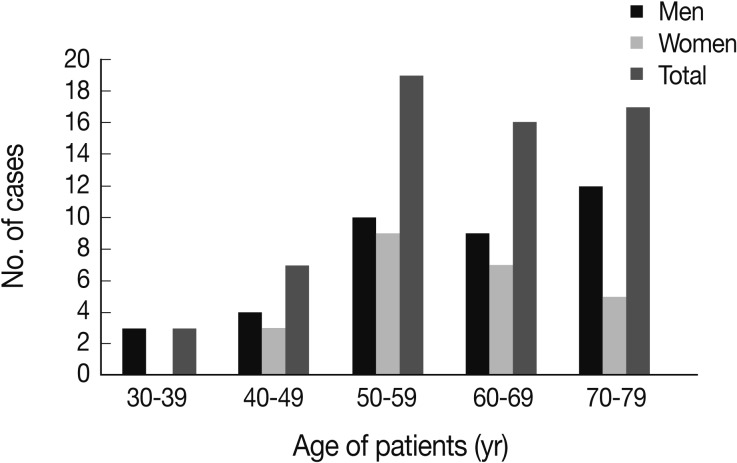
According to the age distribution, the majority of cases developed malignant mesothelioma when patients are in their 50s, followed by those over 70, those in their 60s, 40s and finally in their 30s.
The occupational history was available for only 23 (37.1%) patients out of 62 patients, and among these, only 11 (17.7%) had a job that placed them at risk for asbestos exposure such as construction, automobile repair and production, shipbuilding and repair, or farming (Table 2).
Immunohistochemistry and real-time PCR findings
None of the 62 MM tissue samples studied showed a positive immunohistochemical staining for SV40, while the positive control kidney tissue for each slide was strongly positive (Fig. 2).
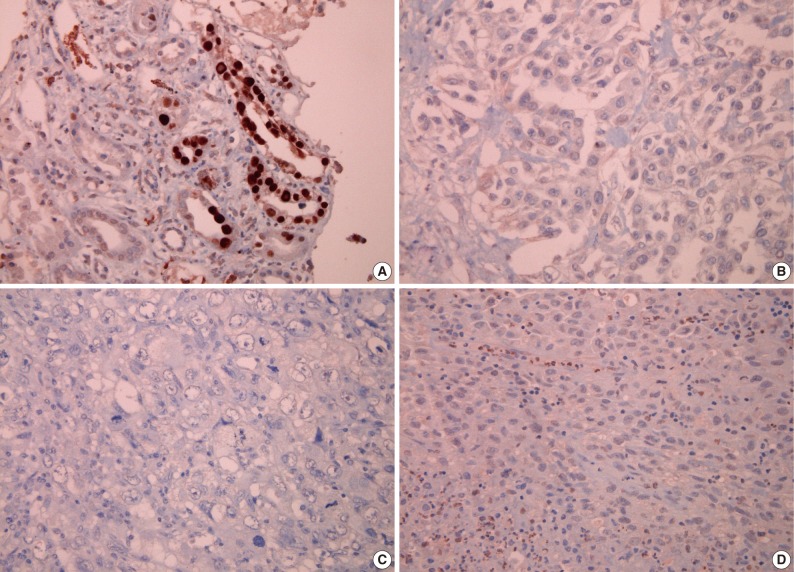
Immunohistochemical findings for simian virus 40 (SV40) antibody; (A) positive control tissue from kidney infected with SV40 shows a positive reaction, while tissue sections from malignant mesothelioma show a negative result for SV40 antibody (B, epithelioid type; C, biphasic type; D, sarcomatoid type).
Of the 36 samples that yielded sufficient DNA following extraction and purification, real-time PCR analysis failed to show SV40 DNA amplification in any case. The mean cycle threshold (Ct) value for the 36 available blocks was 34.133±1.440 and was not significantly different from the negative control (positive control, 28.413±0.476; negative control, 34.437±0.160).
DISCUSSION
The possible association between SV40 and MM has inspired much interest, especially after Carbone et al.16 reported the presence of SV40 in approximately 60% of mesotheliomas. Several subsequent studies have investigated the relation of mesothelioma with SV40, but reported results have varied considerably. One study which investigated the presence of SV40 T-Ag in 18 autopsied MM patients in Japan involved eight positive samples; however, none of these cases were likely to have received a contaminated polio vaccine due to their age.12 A recent review showed that the association of SV40 and MM was positive in only three cases out of 12.6 Another three studies showed that an average of 22% of tumor tissues were positive for SV40.6,10
A strong association between exposure to asbestos and the development of MM has long been established, but there exist cases with unclear etiologies that appear unrelated to asbestos exposure. MM is known to occur in only 10% to 20% of individuals heavily exposed to asbestos, and about 20% of patients lack a history of any exposure.17 In a case-control study, PCR analysis revealed the presence of SV40 DNA in eight of 19 cases of MM among individuals exposed to asbestos, suggesting that SV40 may increase the risk of MM among patients exposed to asbestos.13
In hamsters, intrapleural injection of SV40 virus resulted in the development of MM in 100% of cases within two to six months.18 Additionally, a strong association between asbestos and SV40 was found among hamsters exposed to low levels of asbestos, as only the SV40-infected animals developed MM.19 To understand the roles of both T-Ag and t-Ag in the development of MM in hamsters, t-Ag mutant SV40 virus was injected intracardially into newborn hamsters, after which only one animal developed MM, while the majority developed histiocytic lymphomas.20 While T-Ag binds and deactivates tumor suppressor proteins, t-Ag may play a key role in mesothelial cell transformation and may be necessary to complete inactivation and allow mesothelial cells to enter S phase.11,20
In humans, the role of SV40 in the development of MM is controversial. In total, more than 26 studies have identified SV40 in human mesotheliomas or other human tumors;11 however, many recent investigators have been unable to detect SV40 in human mesothelioma tissues.10,11 In a study by Manfredi et al.,21 of 69 human MM tissues screened, none contained detectable SV40 T-Ag sequences, arguing against the role of SV40 in the development of human MMs.
In this study, no evidence of SV40 was found in any of the MM tissues examined, either by immunohistochemistry or realtime PCR analysis. These results are consistent with those of most recent investigations. The discrepancy found among these different studies may suggest a possible role for geographical differences in the association between SV40 and MM. Alternatively, contamination of laboratory plasmids may have led to false positive results in some cases.22
Occupational and environmental exposure to asbestos is the main causes of MM in Korea.15 Our results do not support an association between MM and SV40 or the proposed function of SV40 as a cofactor to asbestos carcinogenicity. If SV40 is indeed a cofactor for asbestos carcinogenicity, viral transcripts and proteins would have been detectable in the cases examined in our study.
We acknowledge some advantages and limitations of our study. We performed immunohistochemistry and real-time PCR separately to detect SV40 protein and DNA, respectively, in MM tissues. Frozen or fresh tissues are preferred for PCR detection of viral DNA. However, acquiring frozen or fresh MM tissues is impractical due to the infrequency of MM. In addition, our sample size may not have been large enough to detect an association between SV40 and MM in the Korea.
In conclusion, no detection of SV40 in MM tissues suggests that the virus does not have a role in development of MM in Korea; therefore, a more active epidemiologic study for asbestos-related occupational and environmental causes in MM patients is needed.
Acknowledgments
This study was supported by the Korean Ministry of Environment as "The Environmental Health Action Program." The authors gratefully acknowledge financial support by the Korean Ministry of Environment. The authors also would like to thank the members of the Cardiopulmonary Pathologists Society, Korean Society of Pathologists for reporting malignant mesothelioma cases.
Notes
No potential conflict of interest relevant to this article was reported.