Prognostic Significance of BCL9 Expression in Hepatocellular Carcinoma
Article information
Abstract
Background
BCL9 enhances β-catenin-mediated transcriptional activity regardless of the mutational status of the Wnt signaling components and increases the cell proliferation, migration, invasion, and metastatic potential of tumor cells. The goal of this study was to elucidate the prognostic significance of BCL9 protein expression in hepatocellular carcinoma (HCC) patients.
Methods
We evaluated BCL9 protein expression by immunohistochemistry in tumor tissue from 288 primary HCC patients who underwent curative hepatectomy. The impact of BCL9 expression on the survival of the patients was analyzed. The median follow-up period was 97.1 months.
Results
Nuclear BCL9 protein expression was observed in 74 (25.7%) of the 288 HCCs. BCL9 expression was significantly associated with younger age (p=0.038), higher Edmondson grade (p=0.001), microvascular invasion (p=0.013), and intrahepatic metastasis (p=0.017). Based on univariate analyses, BCL9 expression showed an unfavorable influence on both disease-free survival (DFS, p=0.012) and disease-specific survival (DSS, p=0.032). Multivariate analyses revealed that higher Barcelona Clinic Liver Cancer stage was an independent predictor of both shorter DFS (p<0.001) and shorter DSS (p<0.001). BCL9 expression tended to be an independent predictor of shorter DFS (p=0.078).
Conclusions
BCL9 protein expression might be a marker of shorter DFS in HCC patients after curative hepatectomy.
Hepatocellular carcinoma (HCC) has a poor prognosis and surgical resection improves the survival rates for patients. However, the prognosis after surgical resection of HCC remains grave because of a high rate of recurrence.1,2 It is critical to determine which individual patients need adjuvant therapy in order to prevent tumor recurrence after surgical resection. Although there are many reports on histologic parameters for predicting HCC prognosis, molecular markers for HCC recurrence and prognosis could provide additional information.3
BCL9 was identified as a gene overexpressed in a precursor-B-cell acute lymphoblastic leukemia cell line with t(1;14)(q21;q32) translocation4 and it is involved in the Wnt/β-catenin signaling pathway by mediating the recruitment of pygopus to the nuclear β-catenin-TCF complex.5 Upregulation of the Wnt/β-catenin signaling pathway appears to play an important role in the development or progression of certain cancers, including colorectal cancer and HCC.6 Deregulated expression of β-catenin leads to the stabilization of β-catenin and the stabilized β-catenin translocates into the nucleus, where it can serve as a transcriptional factor through binding with the TCF transcriptional factor.7 BCL9 enhances the β-catenin-mediated transcriptional activity regardless of the mutational status of the Wnt signaling components and increases the cell proliferation, migration, invasion, and metastatic potential of tumor cells, while knocking down BCL9 increases the survival outcomes among xenograft mouse models of multiple myeloma and colon cancer.8 Brembeck et al.9 reported that the highly up-regulated BCL9-2 protein was found in almost all of the human colorectal carcinomas, independently of the occurrence of metastasis. A copy number gain on 1q is frequently observed in HCCs.10,11 The BCL9 gene is located at chromosome 1q21 and the regional chromosomal gain represents a primary mechanism in the activation of protooncogenes during HCC progression.12 However, the prognostic significance of BCL9 in HCC remains uncertain.
In the present study, we evaluated BCL9 protein expression by immunohistochemistry in order to elucidate the prognostic significance of BCL9 in HCC patients with long-term follow-up and extensive information on the clinicopathologic characteristics.
MATERIALS AND METHODS
Patients and tissue samples
Primary HCC tissues were collected from 288 patients who were treated with curative hepatectomy at Samsung Medical Center, Seoul, Korea from July 2000 to May 2006 (237 males and 51 females). We defined curative resection as complete resection of all tumor nodules with clear microscopic resection margins and no residual tumors as indicated by a computed tomography scan one month after surgery. The diagnosis was confirmed by histologic examination in all patients. The Institutional Review Board of Samsung Medical Center approved this study. The patients' ages ranged from 17 to 76 years with an average of 52.6. Two hundred and eighteen (75.7%) patients were infected with hepatitis B and 30 (10.4%) with hepatitis C. No viral marker was recognized in 40 (13.9%) patients. None of the patients had received preoperative chemotherapy, transarterial chemoembolization, or radiofrequency ablation. Tumor differentiation was graded histologically according to the criteria of Edmondson and Steiner.13 Microvascular invasion was considered present when at least one or more endothelial cells or the tunica media of the vessel surrounded a neoplastic cell group. Intrahepatic metastasis and multicentric occurrence were defined according to the previously reported criteria.14 Briefly, intrahepatic metastasis is defined as: 1) portal vein tumor thrombi or cancer lesions that have putatively proliferated from a tumor thrombus, 2) groups of cancer lesions that are most abundant adjacent to the largest main lesion and decrease in number with distance from the main lesion, or 3) small solitary cancer lesions located adjacent to the largest main lesion and of the same histological type that are definitely smaller than the main tumor and differentiated to the same degree or less differentiated than the main lesion. Multicentric occurrence is defined as: 1) adenomatous hyperplasia or early HCCs that preserve the existing liver architecture, 2) well differentiated HCCs found at the edge of moderately or poorly differentiated cancer tissues, or 3) multiple HCC lesions that cannot be classified as metastasis based on the above criteria. Tumor stage was determined according to both the American Joint Committee on Cancer (AJCC)15 and the Barcelona Clinic Liver Cancer (BCLC) staging classification.16
Patients were followed by monitoring serum α-fetoprotein levels and three phase dynamic computed tomography scans every three months after surgery. Magnetic resonance imaging was used in order to confirm tumor recurrence in suspected cases. The median follow-up period was 97.1 months (range, 40 to 126 months). Disease-free survival (DFS) was defined from the date of resection until the detection of tumor recurrence. We chose HCC-related mortality (disease-specific death) as the clinical endpoint for survival analysis, defined by Hoshida et al.17 as: 1) tumor occupying more than 80% of the liver, 2) portal venous tumor thrombus proximal to the second bifurcation, 3) obstructive jaundice due to the tumor, 4) distant metastases, or 5) variceal hemorrhage with portal venous tumor thrombus proximal to the first bifurcation. Disease-specific survival (DSS) was defined from the date of resection to HCC-related death. Tumor recurrence was detected in 189 (65.6%) patients and 99 (34.4%) patients died of HCC. Thirty of the 129 deaths in this study were due to non-HCC causes.
Tissues with dysplastic nodule (DN), which is a precancerous lesion of HCC (n=28), were included and DNs were subdivided into low-grade DN and high-grade DN according to the guideline of the International Working Party.18
Histologic sections were examined by two pathologists and representative tumor regions free from necrosis or hemorrhage were marked in formalin-fixed paraffin-embedded blocks. Two 2.0 mm-diameter tissue cores were punched from the marked areas of each block and arranged in recipient paraffin blocks. Two cores of normal liver tissue from 12 patients with metastatic colonic carcinoma of the liver were included in each array block.
Immunohistochemical analysis
Immunohistochemical staining was performed as described elsewhere.19 Antigen retrieval was performed with 0.01 mol/L citrate buffer (pH 6.0) for 30 minutes in a pressure cooker. The sections were incubated overnight at 4℃ with the rabbit polyclonal antibody to BCL9 (ab37305, 1:100, Abcam Inc., Cambridge, MA, USA). The positive control (human colon carcinoma) showed intense nuclear BCL9 expression in cancer cells while no immunoreactivity was observed in the tissue sections used as negative controls, in which the primary antibody was replaced by preimmune rabbit serum. In order to validate the concordance between the tissue microarrays and whole tumor sections, we also detected BCL9 expression for 40 corresponding whole tumor sections randomly chosen from the 288 cases.
Immunohistochemical staining was assessed by two independent pathologists (C.K.Park and J.Hyeon) without knowledge of the patients' characteristics and any discrepancies were resolved by consensus. The sections were scored by combining the proportion and intensity of the stained tumor cells as reported previously.9 The proportion of stained tumor cells was determined semi-quantitatively and each sample was scored on a scale of 0-3 (0, <5%; 1, 5-30%; 2, 31-60%; 3, 61-100%). The staining intensity was classified as 0 (negative), 1 (weak), 2 (moderate), and 3 (strong). The immunoreactive score of each tumor was calculated by multiplication of the scores of the proportion of stained cells and the staining intensity. Duplicate tissue cores for each tumor showed high levels of homogeneity for both the proportion of stained cells and the staining intensity. When there were differences between the duplicate tissue cores, the higher score was taken as the total score.
Statistical analysis
Statistical analyses were performed using SPSS software (SPSS Inc., Chicago, IL, USA). The chi-square test and Fisher's exact test were used for comparison of the variables. Cumulative survival time was calculated by the Kaplan-Meier method and compared by the log-rank test. Univariate and multivariate analyses were based on the Cox proportional hazards regression model. p-values less than 0.05 were considered statistically significant.
RESULTS
BCL9 protein expression in HCC
BCL9 was detected on the cytoplasm in 3-10% of the normal hepatocytes with weak or moderate staining intensity. In HCC, immunoreactivity for BCL9 was observed in the nuclei of tumor cells with or without cytoplasmic expression. We regarded BCL9 as positive when the total score for nuclear immunoreactivity was 1-9. BCL9 protein expression was observed in 74 (25.7%) of the 288 HCCs (Fig. 1A). BCL9 expression was significantly associated with younger age (p=0.038), higher Edmondson grade (p=0.001), microvascular invasion (p=0.013), and intrahepatic metastasis (p=0.017) (Table 1). None of the 28 DNs showed nuclear immunoreactivity for BCL9 (Fig. 1B).

Immunostaining of BCL9 showing nuclear expression in hepatocellular carcinoma (A) and no nuclear expression in dysplastic nodule (B) (horseradish peroxidase stain).

Correlation of BCL9 expression and tumor recurrence with clinicopathologic features in 288 hepatocellular carcinomas
Tumor recurrence was significantly associated with larger tumor size (p=0.011), higher Edmondson grade (p=0.029), microvascular invasion (p=0.001), major portal vein invasion (p=0.038), intrahepatic metastasis (p<0.001), higher AJCC T stage (p<0.001), higher BCLC stage (p=0.004), higher α-fetoprotein level (p=0.002), viral etiology (p=0.004), and liver cirrhosis (p=0.009) (Table 1).
Survival analysis
The DFS and DSS rates for the 288 HCC patients were 42.7% and 78.2% at three years, 36.3% and 71.4% at five years, 30.1% and 67.1% at seven years, and 27.9% and 60.8% at nine years, respectively. Based on univariate analyses, larger tumor size, Edmondson grade III, microvascular invasion, major portal vein invasion, intrahepatic metastasis, higher AJCC T stage, higher BCLC stage, lower albumin level, and higher α-fetoprotein level showed unfavorable influence on both DFS and DSS. Viral etiology and liver cirrhosis showed unfavorable influence on DFS. BCL9 expression showed an unfavorable influence on both DFS (p=0.012) and DSS (p=0.032) (Table 2). The five-year DFS rate of the BCL9-positive group was significantly lower than that of the BCL9-negative group (24.2% vs 39.2%) (Fig. 2A). The median DFS of the BCL9-positive group and the BCL9-negative group were 9.9 and 23.8 months, respectively. The five-year DSS rate of the BCL9-positive group was significantly lower than that of the BCL9-negative group (61.7% vs 73.6%) (Fig. 2B). The median DSS of the BCL9-positive group and the BCL9-negative group were 63.1 and 86.5 months, respectively.

Univariate analyses of disease-free survival and disease-specific survival in 288 hepatocellular carcinomas
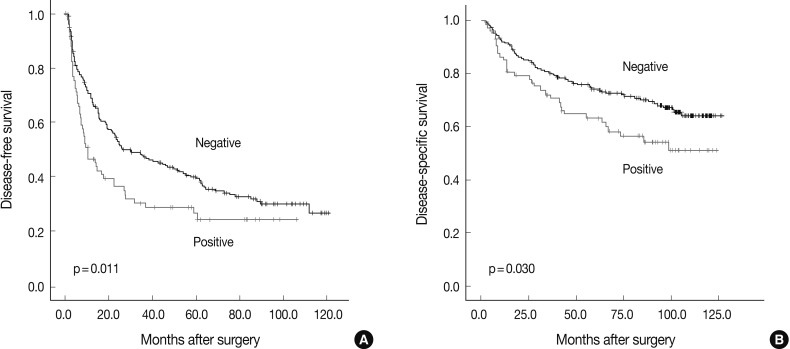
Kaplan-Meier survival curves showing disease-free survival (A) and disease-specific survival (B) for BCL9 expression in 288 hepatocellular carcinomas.
Since tumor size, vascular invasion, intrahepatic metastasis, AJCC stage, and serum albumin level were associated with BCLC stage, we did not perform multiple analyses with these indices in order to avoid potential bias. An evaluation of the significant weight of the serum α-fetoprotein level was not performed due to missing data (n=277). Based on multivariate analyses, higher BCLC stage (p<0.001), viral etiology (p=0.022), and liver cirrhosis (p=0.011) were independent predictors of shorter DFS. BCL9 expression tended to be an independent predictor of shorter DFS (p=0.078). BCL9-positive patients were more likely to suffer from recurrence than BCL9-negative patients (hazard ratio=1.351, 95% confidence interval 0.967-1.888). Higher BCLC stage (p<0.001) was an independent predictor of shorter DSS. However, BCL9 expression was not an independent predictor of DSS (p=0.115) (Table 3).
DISCUSSION
BCL9 is required for Wnt signal transduction at the level of nuclear β-catenin and to exert its function by physically linking pygopus to β-catenin.5 Kramps et al.5 reported that wild-type cells expressed BCL9/legless protein in the nuclei and the nuclear localization of the BCL9/legless appeared to be essential for its signaling activity. Recent reports showed that nuclear BCL9 staining was absent in human normal colon mucosa, but elevated in human colorectal cancer.8,9 In this study, nuclear immunoreactivity for BCL9 was not observed in normal liver or DN tissues. BCL9 protein expression might not be an early event in HCC carcinogenesis. Recent studies have shown that the BCL9-2 protein was significantly up-regulated in human colon adenomas compared with normal colon mucosa,9 which suggests that the deregulation of BCL9 occurs during early stages of colonic carcinogenesis. In colorectal cancer patients, almost all cases showed high BCL9-2 protein expression and it was not correlated with overall survival, indicating that BCL9-2 is not a predictor for advanced tumor stages in colorectal cancer.9
In the current study, we applied tissue microarrays in order to evaluate the prognostic significance of BCL9 protein expression in a large cohort of HCC patients and demonstrated that BCL9 expression was correlated with higher Edmondson grade, microvascular invasion, and intrahepatic metastasis, which suggest the involvement of BCL9 in the pathogenesis of HCC. Tumor recurrence was remarkably associated (p<0.005) with microvascular invasion, intrahepatic metastasis, higher AJCC T stage, higher BCLC stage, higher α-fetoprotein level, and viral etiology. In addition, HCC with BCL9 expression had a lower five-year DFS rate than HCC without BCL9 expression and BCL9 expression tended to be an independent predictor of shorter DFS. HCC with BCL9 expression had a lower five-year DSS rate than HCC without BCL9 expression. These findings indicate that BCL9 is a potential new marker of shorter DFS in HCC after curative resection and the results could help clinicians identify patients at high risk of recurrence.
This study demonstrates, for the first time, that BCL9 protein expression in HCC tissues might be a marker of shorter DFS in HCC after curative hepatectomy in a large number of HCC patients with long-term follow-up. Patients identified as being at high risk of recurrence should be followed closely. Further study is needed in order to examine the mechanism of the actions regarding BCL9 protein expression in HCC prognosis.
Notes
No potential conflict of interest relevant to this article was reported.
