A Solitary Fibrous Tumor with Giant Cells in the Lacrimal Gland: A Case Study
Article information
Abstract
Orbital solitary fibrous tumor (SFT) has recently been proposed as the encompassing terminology for hemangiopericytoma, giant cell angiofibroma (GCAF), and fibrous histiocytoma of the orbit. The lacrimal gland is a very rare location for both SFT and GCAF. A 39-year-old man presented with a painless left upper eyelid mass. An orbital computed tomography scan identified a 1.1 cm-sized well-defined nodule located in the left lacrimal gland. He underwent a mass excision. Histopathologic examination showed a proliferation of relatively uniform spindle cells with a patternless or focally storiform pattern. Dilated vessels were prominent, but angiectoid spaces lined with giant cells were absent. Floret-type giant cells were mostly scattered in the periphery. The tumor was immunoreactive for CD34 and CD99, but negative for smooth muscle actin and S-100 protein. This is the first Korean case of SFT of the lacrimal gland with overlapping features of GCAF, suggesting a close relationship between the two entities.
Solitary fibrous tumors (SFT) are rare spindle cell neoplasms that were first described by Klemperer and Coleman1 as mesenchymal tumors that arise in the pleura. Although the most common site of SFTs is the intrathoracic region, they can also occur at various extrathoracic sites, including the liver, nasal cavity, orbit, thyroid, meninges, skin, kidney, and other organs.2-4 Since Dei Tos et al.5 first reported seven orbital giant cell angiofibromas (GCAFs) as orbital tumors that were distinct from SFTs and giant cell fibroblastomas of the soft tissue, GCAFs have also been found in various extraorbital regions, including the oral cavity, upper back, groin, axillary soft tissue, and parotid gland.6-8 However, some authors reported that SFTs and GCAFs have overlapping clinicopathologic and immunohistochemical features and thus suggested that GCAFs are a variant of SFTs. The overlapping features include their common occurrence in middle-aged adults with an equal gender distribution, their well-circumscribed and variably unencapsulated "patternless" spindle cell lesions with variably collagenized stroma, and their immunopositivity for CD34 and CD99. Some cases of SFTs have angiectoid spaces and multinucleated giant cells in the stroma.9,10 In this report, we present a lacrimal gland SFT with floret-like giant cells that are characteristic of GCAF. The lacrimal gland is a very rare location for SFTs and GCAFs. To our knowledge, only seven cases of lacrimal gland SFTs or GCAFs have been reported.2,11-14 This is the first Korean case of an SFT of the lacrimal gland showing features that overlap with those of GCAFs.
CASE REPORT
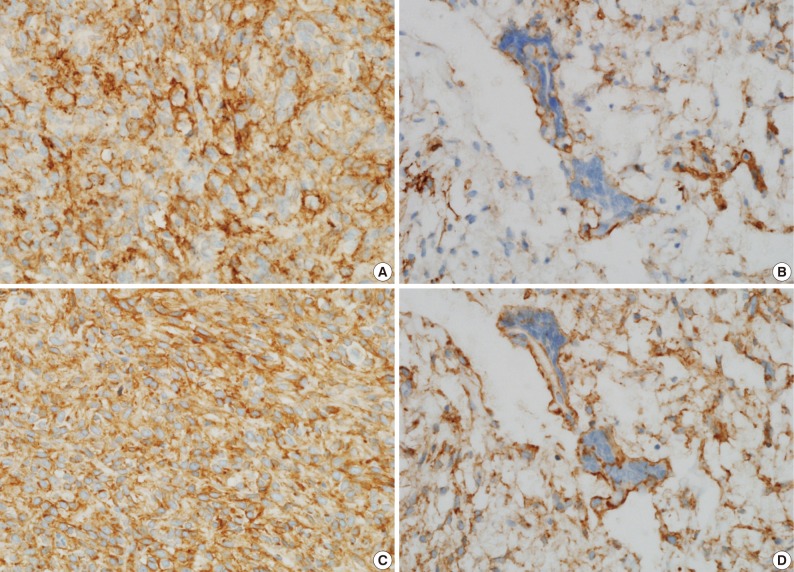
A 39-year-old man presented with a painless left upper eyelid mass that had presented three months previous. An orbital computed tomography scan showed that a 1.1 cm-sized, well-defined nodule with an eccentric nodular enhancement, and an internal low attenuation area was present in the left lacrimal gland (Fig. 1A). An en-block excision was performed under the impression that this mass was a pleomorphic adenoma. The excised mass was well-demarcated, ovoid, and firm, with measurements of 1.5×1.3×0.7 cm. The cut surface had a whitish tan hue and was fibrotic with a multifocal hemorrhage (Fig. 1B). Histopathological examination showed a spindle cell proliferative lesion that was surrounded by the lacrimal gland parenchyma (Fig. 2A). The cellularity was variable and presented as either a patternless or a storiform pattern (Fig. 2B). Collagenized stroma and dense collagen nodules were found around dilated vessels, and perivascular hyalinization was also observed (Fig. 2C). The spindle cells had scant cytoplasm and short, oval spindle nuclei with fine chromatin and inconspicuous nucleoli. Multinucleated giant cells were either observed in the periphery of the mass giant cells or were not identified (Fig. 2D). Mitotic activity was low (only observed in two of ten high magnification fields). Immunohistochemistry demonstrated that the spindle cells were strongly positive, and the multinucleated cell cytoplasm was focally positive for CD34 and CD99 (Fig. 3). Smooth muscle actin and S-100 protein were not detected. The diagnosis of a SFT was made on the basis of the histological findings, which showed that the mass lacked the typical components and immunoprofiles of giant angiofibromas.
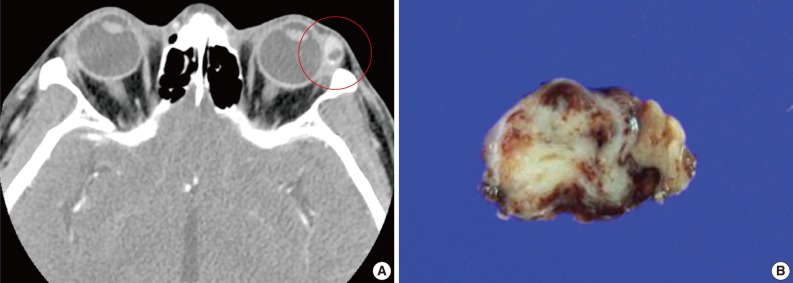
(A) An orbital computed tomography scan shows a 1.1 cm-sized, well-demarcated nodule with an eccentric nodular enhancement and an internal low attenuation area in the left lacrimal gland. (B) The solitary fibrous tumor specimen from the lacrimal gland is a well-circumscribed, whitish tan, firm, and fibrotic nodule with a hemorrhagic lesion.
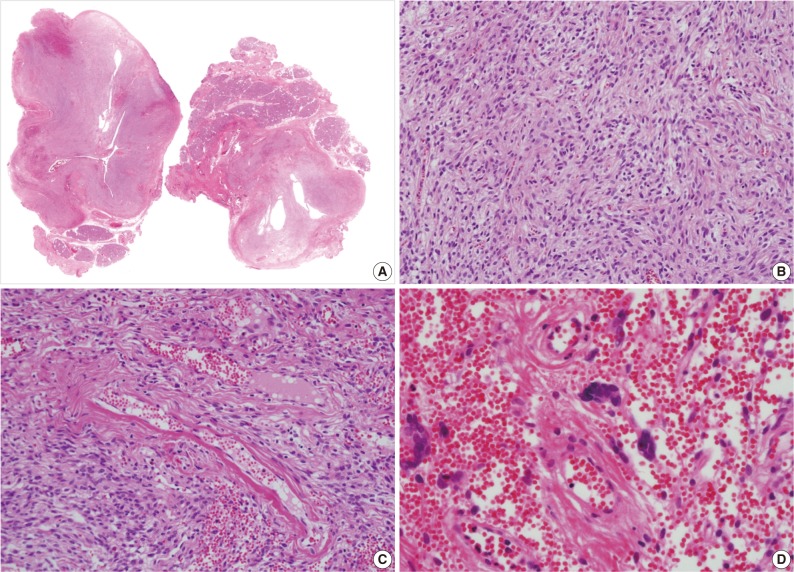
(A, B) Microscopic examination of the lacrimal gland lesion shows proliferation of relatively uniform spindle cells that are either patternless or have a focally storiform pattern. (C) Focal vascular dilatation with collagenized stroma, dense collagen nodules, and perivascular hyalinization is also observed. (D) A small number of floret-type giant cells are scattered at the periphery, but no angiectoid spaces lined with giant cells are found.
DISCUSSION
Although SFTs and GCAFs are listed as separate entities in the current World Health Organization (WHO) classification, it is generally acknowledged that the features of SFTs, GCAFs, hemangiopericytomas (HPC), and lipomatous HPC are commonly shared by a subset of tumors. Traditionally, SFTs are described as having patternless spindle cell tumors that are rich in collagen, and HPCs are described as having thin-walled, branched, or staghorn blood vessels. GCAFs are distinguished from these tumors by the presence of multinucleated giant stromal cells that often line the angiectoid spaces.5,15 These distinct entities also have common immunophenotypes, including positivity for both CD34 and CD99.
The notion that SFTs and GCAFs should be united into one category appears to be gaining popularity among oncologists. In 1999, Hasegawa et al.10 described the presence of giant cells in six of 24 extrapleural SFTs. Guillou et al.7 studied one orbital and nine extraorbital GCAFs and asserted that GCAFs are merely a giant cell-rich variant of SFTs. They observed that GCAFs and SFTs have overlapping morphologic and clinical features. Both GCAFs and SFTs are well-circumscribed, nonencapsulated lesions that occur in middle-aged adults with an equal gender distribution and which can occur either inside or outside of the orbital region, contain a varying number of multinucleated giant cells, and are immunopositive for CD34 and CD99.7,10 Piperi et al.9 also demonstrated a lingual example of a vascular SFT, where an overlapping characteristic with GCAFs included the presence of "floret" cells. Recently, Furusato et al.2 reappraised 41 orbital cases and suggested orbital SFT as an encompassing terminology for HPC, GCAFs, and fibrous histiocytomas. They observed that the majority of cases presented with a prominent vasculature with perivascular fibrosis and were composed of haphazardly disposed fibroblast-like cells with round or oval nuclei, indistinct nucleoli, and variable stromal collagen. In addition, staghorn vessels, giant cells, fat, and ropey collagen were all common features whose statuses varied among tumors and even among different portions of the same neoplasm.2 Most of the cases originated from the orbital soft tissue, and only two cases of lacrimal gland tumors were included.
The lacrimal gland tumor presented here also exhibited a patternless spindle cell proliferation with perivascular fibrosis and giant cells but did not present prominent pseudovascular spaces. Therefore, this tumor showed features that were intermediate between those of SFTs and those of GCAFs. Therefore, this case provides another example that supports the unifying concept. There seems be a morphological spectrum of SFTs whose morphologies vary depending on the site of the tumor. The orbital cases might more often present GCAF-like features than the SFT-like features observed at other sites for unknown reasons. To our knowledge, only seven cases of lacrimal gland SFTs or GCAFs have been reported since the first one was observed in 1996 by Scott et al.14 Among the seven cases, two were Korean (a 24-year-old man and a 26-year-old woman).11 The tumors in these cases were described as having unusual adenofibromatous features without any mention of giant cells. Therefore, this is the first Korean case report of a lacrimal gland SFT with features that overlap with those of GCAF. This study further suggests the existence of a close relationship between SFTs and GCAFs.
SFTs are mostly benign, but some cases have been associated with malignancy.16 In the case described here, the tumor was small (1.5 cm at its largest dimension) and had a low mitotic activity (only observed in two of ten high magnification fields). The tumor did not present necrosis, nuclear pleomorphism, or an invasive growth pattern. England et al.17 suggested histological parameters that indicate the malignancy of SFTs in intrathoracic regions, which include increased cellularity, nuclear pleomorphisms, observation of mitosis in more than four of ten high magnification fields, hemorrhages, and necrosis. Vallat-Decouvelaere et al.16 suggested that nuclear atypia, hypercellularity, mitosis in four of ten high magnification fields, and necrosis be considered as malignant features in extrathoracic SFTs. The morphological criteria for uncertain behavior or low-grade malignant ocular SFTs are proposed to be the presence of cytologic atypia and increased mitotic activity.4 However, the overall outcomes for malignant solitary fibrous tumors in the eyes should be further explored.
Notes
No potential conflict of interest relevant to this article was reported.