Papillary Carcinoma of the Thyroid Gland with Nodular Fasciitis-like Stroma
Article information
Abstract
Papillary thyroid carcinoma with nodular fasciitis-like stroma (PTC-NFS) is a rare variant of PTC. The term 'PTC with fibromatosis-like stroma' has been used as a synonym to describe this variant. It is characterized by extensive proliferation of fibroblasts and myofibroblasts in the tumor stroma, which occurs in up to 80% of the tumors. We herein describe a case of PTC-NFS which developed in a 49-year-old woman with the demonstration of findings of ultrasonography, fine needle aspiration cytology and histological examination of the lesion. To characterize the stromal components, we investigated the expression of several immunohistochemical markers which have been shown to be expressed differently in nodular fasciitis (NF) and fibromatosis (FM). The immunostaining results demonstrated nuclear and cytoplasmic accumulation of β-catenin, cytoplasmic transforming growth factor-β expression and nuclear Smad expression in the stromal cells, suggesting that the stromal cells in this case have similar molecular profiles to those of FM rather than NF.
Papillary thyroid carcinoma (PTC) is known to have several morphologic variants. PTC can occasionally manifest with extensive proliferation of the stroma, resembling fibroblastic/myofibroblastic proliferative lesion in the soft tissue. This rare variant of PTC has been described with the terms 'PTC with nodular fasciitis-like stroma (PTC-NFS)' or 'PTC with fibromatosis-like stroma (PTC-FMS).' Histologically, it consists of stromal components rich in spindle cells, occupying 60-80% of tumors, and small foci of epithelial components showing typical features of conventional PTC. Ultrastructural and immunohistochemical findings have revealed that the spindle cells in the tumor stroma have characteristics of myofibroblasts.1
We herein describe a case of PTC with PTC-NFS that developed in a 49-year-old Korean woman. Recent studies have introduced several immunohistochemical markers, which are potentially useful in the differential diagnosis of nodular fasciitis (NF) and fibromastosis (FM). We investigated immunoprofiles of these molecules in the present case to assess molecular characteristics of the tumor stroma.
CASE REPORT
A 49-year-old woman presented with a lump in her neck, which had gradually increased in size during the previous two months. Physical examination revealed a firm nodular mass in the right lobe of the thyroid gland. Her past medical history was unremarkable. Thyroid function tests were within normal range. Ultrasonography revealed a round, relatively well-defined, mixed echoic nodule in the right lobe. The nodule had a markedly hypoechoic portion in the lateral side and had a slightly hypoechoic portion in the medial side. Color Doppler imaging showed increased blood flow within the markedly hypoechoic portion of the nodule (Fig. 1).
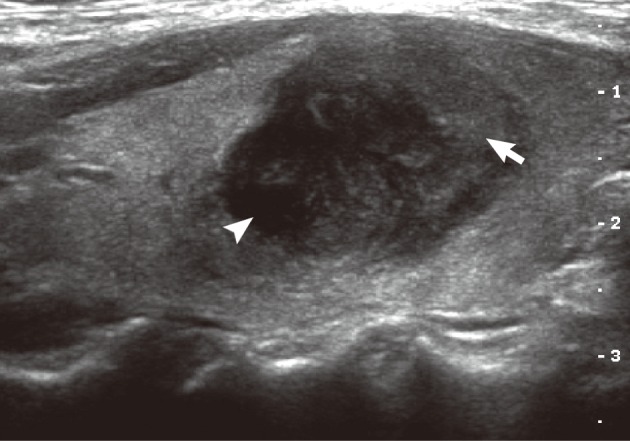
Ultrasonography demonstrates a mixed echogenic round mass, which consists of a markedly hypoechoic portion (arrow head) and a slightly hypoechoic portion (arrow).
The fine needle aspiration in this nodule exhibited clusters of cuboidal cells with features of papillary carcinoma. The smear also contained spindle cell groups with non-cohesive arrangement among the tumor cells of PTC. The spindle cells were in a pale to eosinophilic background with associated lymphocytic infiltrate. They had vesicular nuclei, small nucleoli, and indistinct cytoplasmic membrane. Neither significant atypia nor mitotic figures were identified (Fig. 2). Total thyroidectomy with central lymph node dissection was performed.

Fine needle aspiration reveals bland looking spindle cells with a non-cohesive arrangement in an eosinophilic background (Papanicolaou stain).
Grossly, the right lobe of the thyroid gland showed a well-circumscribed yellow to white, partially encapsulated mass, measuring 2.3×2.0×1.8 cm. Histologically, the tumor consisted of two distinct components: stromal and epithelial. The stromal component consisted of spindle-shaped cells arranged in interlacing fascicles and an abundant fibromyxoid matrix accompanied by thick collagen fibers. The stromal cells had neither nuclear atypia nor mitotic figures. Occasional lymphocytic infiltration or extravasated red blood cells were also identified in the center and periphery of the tumor stroma. Epithelial components had features of PTC (Fig. 3). The tumor was confined to the thyroid parenchyma. Metastatic tumor tissue was detected in four of five resected central lymph nodes without stromal components. Immunohistochemically, the spindle cells exhibited focal cytoplasmic staining with desmin and diffuse cytoplasmic stain with smooth muscle actin. In contrast, they showed negative staining with cytokeratin and thyroglobulin. These findings are consistent with previous reports of PTC-NFS.2
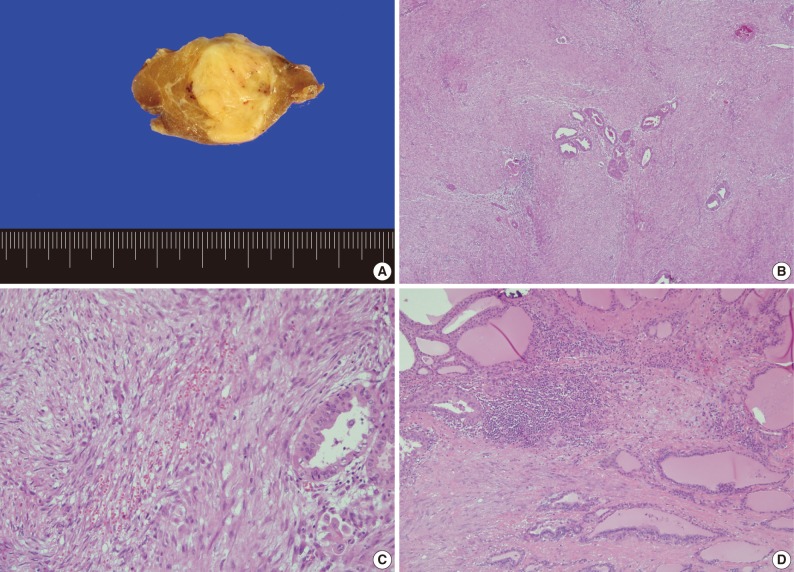
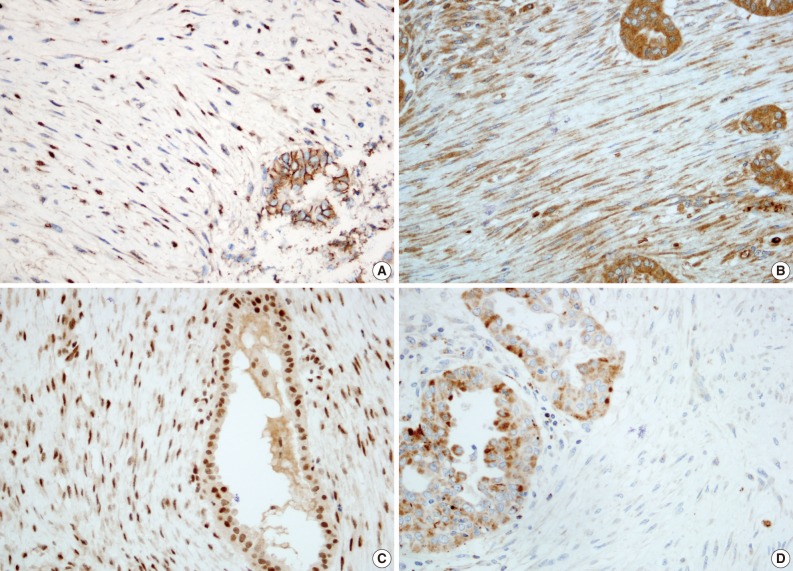
Gross and microscopic findings. (A) The cut surface shows a well-demarcated, yellow to white nodule in the thyroid parenchyma. (B) The tumor consists of extensive stromal proliferation with small foci of papillary carcinoma. (C) The stromal component shows spindle cells arranged in interlacing fascicles, lymphocytic infiltration and extravasated red blood cells. (D) The periphery of the tumor stroma also shows lymphocytic infiltration.
We further performed immunohistochemical analyses of β-catenin, transforming growth factor-β (TGF-β), Smad-2 and -4, and matrix metalloproteinase (MMP)-3 and -9, which have been reported to be useful in distinguishing FM from NF.3 The immunostaining results for the stromal spindle cells are summarized in Table 1. The spindle cells showed nuclear and cytoplasmic β-catenin expression and cytoplasmic TGF-β expression. Smad-2 and -4 were also localized in the nuclei of the spindle cells. In contrast, the spindle cells lacked MMP-3 expression and showed scant cytoplasmic staining with MMP-9 (Fig. 4).
DISCUSSION
PTC-NFS has recently been described as a variant of PTC. The importance of recognizing this variant of PTC is that, when a fibroproliferative lesion is detected in the thyroid gland, a careful search should be made for PTC. It has been previously shown that fine needle aspiration in this disease can only contain spindle cells.4 Furthermore, recognition of this variant is particularly important in the differential diagnosis from extensive fibrosis observed in other malignancies of the thyroid glands showing aggressive clinical course, such as diffuse sclerosing variant of PTC and carcinosarcoma. The stroma in the diffuse sclerosing variant of PTC is rich in lymphocytic infiltrate and psammoma bodies, but fibroblastic proliferation is rarely seen. Carcinosarcoma can be differentiated by the detection of obvious malignant features of spindle cells in the tumor stroma.5
To our knowledge, 24 cases of PTC-NFS have been reported in the literature. In both present and previous reports, the clinical features of this variant were similar to those of conventional PTC. The age at presentation ranged from 20 to 82 years (mean, 45 years) with a female preponderance (3:1). Most patients presented with palpable neck mass, and the size of the mass ranged from 2 to 9 cm. Metastasis to lymph nodes occurred in six of 24 patients, but distant metastasis has not yet been reported. The prognosis of patients with PTC-NFS seems to be similar to that of patients with conventional PTC. However, there have been some opinions that the follow-up data is insufficient to determine the clinical course of this disease.1,6-8
On ultrasonography, NF presents as an isoechoic or slightly hypoechoic mass with variable vascularity.9 There has been one earlier report which commented on the ultrasonographic findings of PTC-NFS. The lesion in the previous case showed homogeneous mild hypoechogeneity with scant vascularity, suggesting similar ultrasonographic findings with those of NF.8 The lesion in the present case consisted of a markedly hypoechoic portion and a slightly hypoechoic portion, with the markedly hypoechoic portion showing an increased blood flow. It is considered that the mild hypoechogenic portion in the present case represents the stromal components of PTC-NFS, while the markedly hypoechoic portion represents the carcinomatous components.
The stroma in this variant of PTC has been called NFS and FMS based on histological features in soft tissue. Some histological criteria for the differential diagnosis of NF and FM include the presence of mitotic activity, the location of inflammatory infiltration and the growth pattern.10 In many previous reports, the stroma of PTC-NFS has been described to show a well-circumscribed growth pattern, paucity of mitotic figures and infiltration of lymphocytes and red blood cells, although the location of inflammatory foci was not described precisely.11,12 Only two cases of PTC-NFS with an infiltrative tumor stroma have been reported.5,13 The stroma in the present case showed paucity of mitotic figures and a well-circumscribed growth pattern. Infiltration of lymphocytes and red blood cells was shown in the periphery and in the center of the lesion. The histological findings of the stroma in this case were not typical of NF or FM.
The differential diagnoses of NF and FM are often problematic as they often have overlapping histological features. A number of studies have been performed to help differentiate between an NF and an FM diagnosis and suggested that β-catenin, TGF-β, Smad, and several types of chemokine ligands and MMPs could be useful markers in a differential diagnosis.3,14 We performed inmmunohistochemical staining with β-catenin, TGF-β/Smads, and MMP-3 and -9, based on previous studies. The results of the immunohistochemistry demonstrated that the tumor stroma observed in the present case had similar molecular profiles to those of FM; therefore, the term 'PTC-FMS' may be more appropriate.
In conclusion, we have described a case of PTC-NFS with the demonstration of findings by ultrasonography, fine needle aspiration cytology and a histological examination of the lesion. To characterize the stromal components, we investigated the expression of several immunohistochemical markers which have been shown to be differently expressed in NF and FM. Our findings suggest that the spindle cells in the tumor stroma in this case have similar molecular profiles to those of FM rather than NF.
Notes
No potential conflict of interest relevant to this article was reported.