Bronchial Schwannomas: Clinicopathologic Analysis of 7 Cases
Article information
Abstract
Background
It has long been recognized that bronchial schwannomas are extremely rare. As such, diagnosing tumors in this extraordinary location can sometimes be problematic.
Methods
We reviewed seven cases of bronchoscopically or surgically resected endobronchial schwannomas and evaluated their clinical and pathologic features.
Results
The present study included five female and two male patients, with ages ranging from 16 to 81 years (mean age, 44.9 years). The clinical presentation varied according to tumor size and location. Patients with more centrally (trachea or main bronchus) located tumors experienced respiratory symptoms (80%) more often than patients with more peripherally (lobar or segmental bronchus) located tumors (0%). Histologically, the tumors were composed of spindle cells that stained with S100 protein. Some of the tumors showed typical Antoni A areas with Verocay body formation. Five of six patients (83.3%) underwent complete tumor removal by rigid bronchoscopy.
Conclusions
Pathologists should consider endobronchial schwannoma in the differential diagnosis of a spindle cell tumor involving the bronchus. Additionally, our results showed that rigid bronchoscopy is an effective tool for tumor removal in endobronchial schwannoma patients.
Schwannomas are benign tumors that arise from peripheral, spinal, or cranial nerves, excluding the optic and olfactory nerves.1 They may occur nearly anywhere in the body, but the most common sites for schwannomas are the head, neck, and flexor surfaces of the upper and lower extremities.2,3 The histologic diagnosis of a schwannoma is not particularly difficult. However, schwannomas rarely present as endobronchial lesions. These tumors generally present late, and most patients are asymptomatic. Since this disease is rare and few typical signs are associated with it, preoperative diagnosis is difficult.
Surgical treatment has been the first choice for benign endobronchial tumors, but in recent years, bronchoscopic tumor removal for benign endobronchial tree tumors has been reported to be effective.4-6 However, there is limited information about bronchoscopic tumor removal of endobronchial schwannomas.
In this study, we retrospectively review seven cases of surgically or bronchoscopically resected bronchial schwannomas and discuss the clinicopathological characteristics, diagnostic considerations, and treatment options for these tumors.
MATERIALS AND METHODS
Patients
During a 19-year period (1995-2013), a total of seven patients with endobronchial schwannomas were bronchoscopically or surgically treated at Samsung Medical Center in Seoul, Korea. Each patient's clinical information, including age at the time of diagnosis, sex, clinical presentation, notable past history, and radiologic and bronchoscopic findings, were obtained from our electronic medical record database. This study was approved by the Institutional Review Board of Samsung Medical Center (SMC 2013-04-095).
Tumor classification according to its location
We classified tumors into central or peripheral type according to tumor location. Our definition differed from the classification of Kasahara et al.,7 which classified tumors located in the trachea or in the proximal bronchus and that are visible by bronchofiberscopy as the central type. In the current study, we simply considered the tumor location regardless of bronchoscopic accessibility. The tumor was classified as being centrally located when the tumor was located in the trachea or main bronchus. When the tumor was located in the lobar bronchus or segmental bronchus, we considered the tumor to be peripherally located.
RESULTS
A summary of the clinical, radiologic, bronchoscopic features, and treatment details is presented in Table 1.
Clinical features
There were five female and two male patients, showing female predominance (57.1%), and the patients' ages ranged from 16 to 81 with a mean age of 44.9 years. Each patient's history and clinical presentation is described below. Three cases (42.8%) involved the main bronchi, one case (14.3%) involved both the carina and main bronchus, one case (14.3%) involved the carina, one case (14.3%) involved the lobar bronchus, and one case (14.3%) involved the segmental bronchus. Clinical presentations included cough, dyspnea, hemoptysis, pleuritic chest pain, and postobstructive pneumonia. One patient was asymptomatic, and the tumor was detected during a routine chest radiographic examination. Among five cases with centrally located schwannomas (carina and main bronchus), four patients (80%) experienced respiratory symptoms such as dyspnea. The patient with carinal involvement also had hemoptysis. However, none of the patients with peripherally located tumors (lobar and segmental bronchus) experienced this symptom. The patient with lobar bronchus involvement had pleuritic chest pain. The patient with segmental bronchus involvement of the tumor was asymptomatic. The duration of symptoms between onset and presentation ranged from one month to 19 months (average, 7 months), with the exception of the asymptomatic patient.
Radiologic features
The tumor size varied from 1.5 to 4 cm (mean size, 2.7 cm). A variety of radiologic impressions were encountered in these cases. These included malignant tumors, such as squamous cell carcinoma, carcinoid tumor, adenoid cystic carcinoma, mucoepidermoid carcinoma, and metastatic breast cancer, as well as benign tumors such as leiomyoma, cylindroma, inflammatory pseudotumor, and schwannoma. Two patients (cases nos. 3 and 5) had pneumonia (Fig. 1A, B). In case no. 2, the tumor showed lobulation with extraluminal extension (Fig. 1C), and the radiologist had a suspicion of malignant tumor. In case no. 4, the patient had a past medical history of breast cancer, and an endobronchial nodule was revealed on computed tomographic (CT) scan (Fig. 1D). Both the clinician and the radiologist suspected metastatic breast cancer. An impression of schwannoma was noted in only one case. In case no. 3, the radiologist placed the impression of schwannoma on the differential diagnosis list.
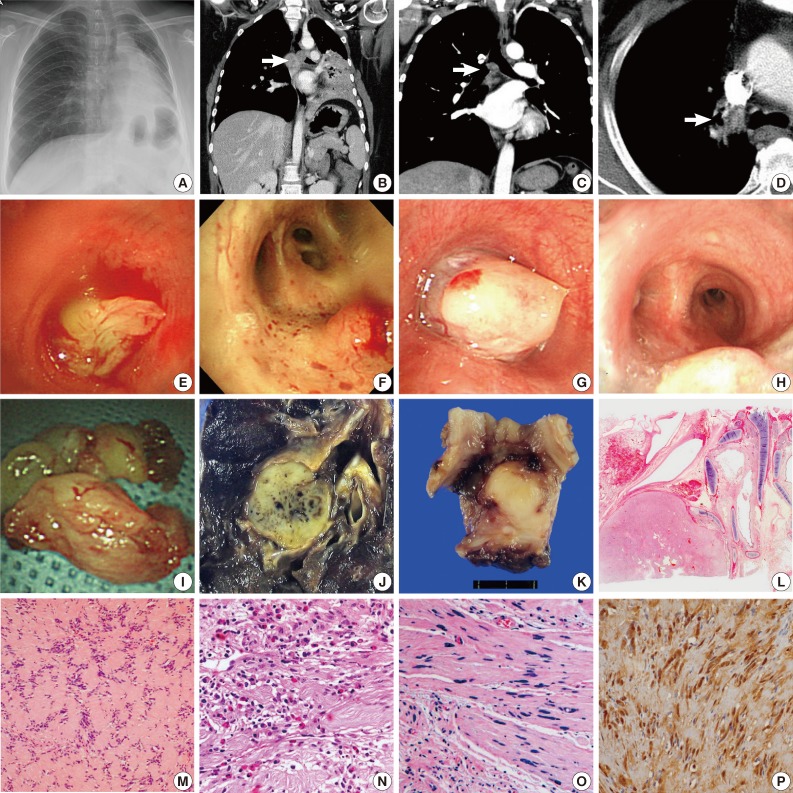
(A) Pneumonia observed on chest X-ray. (B) Corresponding computed tomography scan showing a polyploid bronchial mass and postobstructive pneumonia. (C) A tumor located in the carina with lobulated contour and extraluminal extension. (D) An endobronchial nodule. (E) A lobulated mass completely obstructing the bronchial lumina. (F) A well-circumscribed, raised lesion. (G) A whitish, round mass almost completely obstructing the bronchial lumen. (H) Bronchoscopy showing a whitish, smooth surfaced tumor at the carina. (I) A bronchoscopically resected tumor showing a smooth, lobulated contour. (J) Patient no. 6 shows segmental bronchial involvement and undergo a left lower lobectomy. (K) Surgical resection of a recurred tumor is performed in case no. 2. (L) Bronchial cartilage and a well-circumscribed tumor at low magnification. (M) Tumor showing a palisading arrangement of nuclei (Verocay bodies). (N) Eosinophilic infiltration and collagen deposition is noted in some cases. (O) Tumor with ancient change showing degenerative cells with pleomorphic, hyperchromatic nuclei. (P) Positive immunohistochemical staining for S100 protein can help make a correct diagnosis.
Intervention
Bronchoscopy was performed in every case. Even in the patient with the tumor located in the segmental bronchus (case no. 6), tumors were observed by bronchoscopy. The tumors were described as lobulated (Fig. 1E), well-circumscribed (Fig. 1F), round (Fig. 1G), or raised/smooth surfaced (Fig. 1H) lesions. Visible vessels were also noted in one case. With the exception of one case, all patients had the initial treatment of rigid bronchoscopic tumor removal (Fig. 1I). Only the patient with segmental bronchus tumor involvement (case no. 6) had surgical resection as the initial treatment (Fig. 1J). Among the six patients with initial bronchoscopic resection, five patients had complete tumor removal without recurrence. One patient (case no. 2) experienced tumor recurrence. The patient underwent surgical resection for the recurred tumor (Fig. 1K). None of the patients died of bronchial schwannoma.
Histological features
Histologically, the tumors were visualized as relatively well-circumscribed masses with or without adjacent bronchial cartilage (Fig. 1L). All cases showed similar microscopic characteristics of schwannoma: cellular areas (Antoni type A), more loosely structured areas (Antoni type B), and formation of Verocay bodies (Fig. 1M). Cytologically, tumor cells were bland-looking spindle cells with indistinct cell borders and club-shaped nuclei. Hemorrhage, collagen deposition, and eosinophilic infiltration (Fig. 1N) were also noted in some cases. Two cases (cases nos. 4 and 5) exhibited ancient change (Fig. 1O). On light microscopy, differential diagnosis included leiomyoma and inflammatory myoblastic tumor. Every case demonstrated positivity for S100 protein immunohistochemical staining (Fig. 1P), which helped confirm the diagnosis of endobronchial schwannoma.
DISCUSSION
Since Verocay and Antoni described the histological aspects of schwannoma in the early 1900s, pathologists have been well aware of the histopathologic features of this tumor. A schwannoma is a benign peripheral nerve sheath neoplasm that is composed almost entirely of Schwann cells. Microscopically, the tumor consists of distinctive architectural patterns: cellular "Antoni type A" areas and loosely structured "Antoni type B" areas. Additionally, these tumors have another characteristic feature: "Verocay bodies." A typical Verocay body consists of a stacked arrangement of horizontal rows of palisaded nuclei that are separated by areas of acellular pink zones composed of cytoplasmic processes of the Schwann cells.8
While schwannoma involving various parts of the body is a relatively frequently encountered histologic diagnosis for pathologists, it rarely presents as an endobronchial lesion. Therefore, a clinician may not suspect a schwannoma in a patient with an endobronchial lesion. Benign endobronchial tumors themselves are uncommon, and of these, endobronchial schwannoma is a rare entity, constituting approximately 2% of benign tracheobronchial tumors.9 Although rare, schwannomas can occur in any area of the tracheobronchial tree, with intraluminal or extraluminal extensions.7,10,11
The clinical presentation of endobronchial schwannoma varies and depends on the tumor location, size, and degree of bronchial obstruction. Symptoms include dry or productive cough, fever, hemoptysis, dyspnea, and postobstructive pneumonia, and any one of these can be the first sign of bronchial schwannoma.12
As described above, the clinical symptoms of our patients included cough, fever, hemoptysis, dyspnea, and pleuritic chest pain. Four out of five patients (80%) with more central tumor locations (carina and main bronchus) experienced dyspnea, while neither of the two patients with peripheral locations (lobar and segmental bronchus) had this symptom. Notably, the patient with the most peripherally located tumor (anterior segmental bronchus of the left lower lobe anterobasal segment) was asymptomatic, and the tumor was only discovered on a routine chest radiograph. The duration of symptoms ranged from one to 19 months, with an average of seven months, excluding the asymptomatic patient.
Since symptoms are nonspecific, a diagnosis of schwannoma cannot be made on the basis of clinical presentation. Pre-operatively, several other differential diagnoses for the endobronchial lesion may be considered, including malignant lesions. In our cases, the clinical and radiologic impressions included malignant lesions such as squamous cell carcinoma, adenoid cystic carcinoma, and small cell carcinoma, as well as benign lesions including inflammatory pseudotumor, leiomyoma, and neurogenic tumor. In addition, in three of our cases, radiologists included the possibility of malignant tumor on CT scan. As clinical and radiologic impressions vary, and CT imaging cannot be used to differentiate the nature of the tumor, biopsy is required in most cases.
After a biopsy specimen is obtained, the differential diagnoses can be narrowed into fewer entities by pathology. However, assigning a definite diagnosis on the basis of light microscopy alone is difficult. Initially, tumors that consist of spindled cells, such as leiomyoma, inflammatory myofibroblastic tumor, and meningioma, might be in the differential diagnoses list. One of our patients had a previous biopsy diagnosis of inflammatory pseudotumor at an outside hospital. Although rare, a variety of spindle cell tumors can occur in the tracheobronchial tree, and all of those can be in the differential diagnosis list.
Leiomyoma constitutes around 2% of benign lung tumors, and 45% of pulmonary leiomyomas are endobronchial.13 Patients with endobronchial leiomyoma usually experience respiratory symptoms due to partial or complete airway obstruction.14 On microscopy, leiomyoma are composed of bundles and whorls spindle cells with abundant elongated eosinophilic cytoplasm. The tumors show positivity for desmin and smooth muscle actin, but not for S100 protein.
Inflammatory myofibroblastic tumor is a rare tumor that is usually located in the lung, but can sometimes involve the upper respiratory tract.15 Histologically, the tumor consists of uniform, bland, fibroblast-like spindle cells arranged in fascicles or in a storiform pattern. Characteristically, the tumor is accompanied by an inflammatory infiltrate of plasma cells.16
Meningioma rarely occurs in the pulmonary region, with approximately thirty reports of primary pulmonary meningiomas in the literature.17 These tumors can contain fascicularly arranged tumor cells resembling fibroblasts.17 Positivity for epithelial membrane antigen is not pathognomic, but it is expressed in meningiomas and can be helpful in differential diagnosis.18
In the diagnostic process, the identification of the characteristics of schwannoma, including typical Antoni A formation and Verocay bodies in hematoxylin and eosin stains and S100 positivity aids in confirming the correct diagnosis of schwannoma.19 Although schwannomas are histologically benign, the clinical course depends on the tumor location, size, and degree of bronchial obstruction.12 Kasahara et al.7 reported a case of schwannoma leading to patient death with a complication of pneumonia. Considering that schwannoma can lead to other complications, proper management based on each patient's condition is required.
Until recently, the standard treatment for endobronchial schwannoma has been surgical resection. However, bronchoscopic treatment has been recently utilized for benign tracheobronchial tumors, and has been shown to be a safe and effective tool.4,5 However, to date, there is only limited information in the literature on bronchoscopic removal of endobronchial schwannoma.6
In our cases, aside from one patient with a segmental bronchial tumor, six patients had bronchoscopic tumor removal as the initial treatment. Five of six patients (83.3%) had successful removal of the tumor without recurrence, revealing a high efficacy of bronchoscopic removal of endobronchial schwannoma. Unfortunately, this procedure cannot always achieve complete resection. In case no. 2, the patient underwent rigid bronchoscopic tumor removal at an outside hospital, but residual tumor was observed a month after the intervention, and she required surgery for complete resection of the tumor. Our study revealed that rigid bronchoscopy is a very effective tool for the management of schwannoma, but also showed that it cannot promise complete resection. Therefore, before performing a bronchoscopic intervention, clinicians and surgeons need a thorough evaluation of resectability considering tumor size and location. In asymptomatic patients, watchful waiting can also be an option.
In conclusion, although endobronchial schwannoma is rare, awareness of the possibility of schwannoma involving the bronchus might be helpful in making a correct diagnosis. After diagnosing the tumor, proper management based on each patient's clinical setting is required. In addition, our study revealed successful results following bronchoscopic removal of endobronchial schwannomas.
Notes
No potential conflict of interest relevant to this article was reported.