Clinicopathological Analysis of Hepatocellular Adenoma According to New Bordeaux Classification: Report of Eight Korean Cases
Article information
Abstract
Background
Hepatocellular adenoma (HCA) is a rare benign tumor of the liver. A subtype classification of HCA (hepatocyte nuclear factor 1α [HNF1α]-mutated, β-catenin-mutated HCA, inflammatory HCA, and unclassified HCA) has recently been established based on a single institutional review of a HCA series by the Bordeaux group.
Methods
We used histologic and immunohistochemical parameters to classify and evaluate eight cases from our institution. We evaluated the new classification method and analyzed correlations between our results and those of other reports.
Results
Seven of our eight cases showed histologic and immunohistochemical results consistent with previous reports. However, one case showed overlapping histologic features, as previously described by the Bordeaux group. Four cases showed glutamine synthetase immunohistochemical staining inconsistent with their classification, indicating that glutamine synthetase staining may not be diagnostic for β-catenin-mutated HCA. HNF1α-mutated HCA may be indicated by the absence of liver fatty acid binding protein expression. Detection of amyloid A may indicate inflammatory HCA. HCA with no mutation in the HNF1α or β-catenin genes and no inflammatory protein expression is categorized as unclassified HCA.
Conclusions
Although the new classification is now generally accepted, validation through follow-up studies is necessary.
Hepatocellular adenoma (HCA) is a benign hepatic tumor with a low risk of malignant transformation and a known association with oral contraceptives.1 The incidence of HCA in women with long-term use of oral contraceptives is less than 4 per 100,000/yr.2 Use of oral contraceptives or anabolic steroids has been proposed as a major risk factor for HCAs1,2 although diabetes, glycogen storage disease, and other metabolic disorders are also risk factors.3
There are few reported cases of HCA in Korea. This might reflect the limited use of oral contraceptives in Korea4 due to fear of side effects and the prevalence of tubal ligation and vasectomy during the 1960s and 1970s as part of government-funded family planning.5
Recent studies have shown that HCAs are a group of genetically heterogeneous tumors6,7 that are classified into four subtypes according to their genetic and phenotypic characteristics as follows: hepatocyte nuclear factor 1α (HNF1α)-mutated HCA (H-HCA), β-catenin-mutated HCA (β-HCA), inflammatory HCA (I-HCA), and unclassified HCA.8
Approximately 35-40% of HCAs are H-HCAs, which show marked steatosis but lack cytological atypia and inflammatory infiltrate. This subtype can be identified by the absence of liver fatty acid binding protein (L-FABP) expression on immunohistochemistry because the FABP1 gene is positively regulated by HNF1α. This type of adenoma is associated with a biallelic inactivating mutation of the HNF1α gene.9 Although most of these mutations are somatic, rare germline mutations associated with adenomatosis have been reported.10
With the exception of unclassified HCA, β-HCA is the least common subgroup and accounts for approximately 10-15% of HCAs.11,12 β-HCA has the highest percentage of male patients6,13 and shows cytological atypia, an acinar pattern, and a lack of steatosis. Overexpression of β-catenin and glutamine synthetase can be demonstrated on immunohistochemistry. β-HCAs transform more often than other subtypes but rarely transform malignantly.14 Immunostaining for glutamine synthetase, which is a target of β-catenin,15 is believed to reflect β-catenin mutation.16 Hepatocellular carcinomas (HCCs) tend to be associated with this subtype, particularly in male patients who have received the administration of male hormones, or have glycogenosis or familial polyposis.6,8,11
I-HCA was previously known as telangiectatic focal nodular hyperplasia, but has recently been classified as an HCA subtype.17,18 More than 50% of HCAs are inflammatory HCAs, which reveal an inflammatory infiltrate, sinusoidal dilatation or congestion, and thick-walled arteries. I-HCAs are associated with alcohol consumption and a high body mass index (BMI).13 Two inflammation-related markers, serum amyloid A (SAA) and C-reactive protein, are used to detect this subtype.
Unclassified HCA is the least common subtype, accounting for 5-10% of HCAs, and does not show mutations in the HNF1α or β-catenin genes or inflammatory protein expression.13
We evaluated eight HCA cases from our archives using the histochemical and immunohistochemical methods of the new classification system and compared our results with those of previous reports.
MATERIALS AND METHODS
Hematoxylin and eosin-stained slides and formalin fixed paraffin embedded blocks of eight cases of HCA were retrieved from the archives of the Department of Pathology, Korea University Medical Center and Seoul National University Hospital. The hematoxylin and eosin slides were reviewed, and immunohistochemical stains were performed on the formalin fixed paraffin embedded blocks as followed.
Interpretation of histologic findings
Hematoxylin and eosin-stained slides of each case were reviewed independently by two authors (H.K. and N.H.W.) and a consensus was reached after discussion. The histological characteristics of the tumors were evaluated with emphasis on steatosis, sinusoidal dilatation, inflammatory infiltrate, cytological atypia, acinar growth pattern, telangiectasia, and peliosis. The percentage of steatotic area was recorded. Histological parameters were recorded as negative if less than 10% of the tumor showed the corresponding morphology.
Immunohistochemistry
Immunohistochemical stainings was performed using the following antibodies: L-FABP (1:50, rabbit polyclonal) from Abcam (Cambridge, MA, USA); β-catenin (1:50, mouse monoclonal), SAA (1:50, mouse monoclonal), and glutamine synthetase (GS; 1:200, mouse monoclonal) from BD Biosciences (San Diego, CA, USA); CD3 (1:100, mouse monoclonal), CD20 (1:100, mouse monoclonal), p53 (1:50, mouse monoclonal) and Ki-67 (1:50, mouse monoclonal) from Dako (Carpentaria, CA, USA); and glypican3 (1:50, mouse monoclonal) from Cell Marque (Rocklin, CA, USA).
Cytoplasmic staining of normal hepatic parenchyma served as a positive control for L-FABP immunostaining. Membranous staining of normal hepatic parenchyma served as a positive control for β-catenin immunostaining. Focal cytoplasmic staining in the perivenular area of normal hepatic parenchyma served as a positive control for GS immunostaining.
Diffuse cytoplasmic granular staining with or without membranous staining was recorded as a positive SAA immunostaining result. Focal or faint SAA immunostaining was regarded as negative result. Cytoplasmic staining of normal hepatic parenchyma served as a positive control for SAA immunostaining.
Immunohistochemical staining and histology were independently interpreted by two authors (H.K. and N.H.W.) and a consensus was reached after discussion.
Hepatocellular adenoma subtype classification
The recent HCA classification system proposed by the Bordeaux group was used.8 The histologic and immunohistochemical characteristics for each subtype are described below. Each case was categorized based on combined clinical, histologic, and immunohistochemical parameters.
HNF1α-mutated hepatocellular adenoma
The histologic characteristics of H-HCA are marked steatosis and an absence of cytological abnormalities and inflammatory infiltrate.6,7,13 This subtype shows negative staining for L-FABP because HNF1α positively regulates FABP1 in normal liver tissue. Diffuse or focal cytoplasmic staining was interpreted as a positive result for L-FABP immunostaining. The presence of steatosis and negative L-FABP immunostaining were indicators of H-HCA.
β-Catenin mutated hepatocellular adenoma
The histological characteristics of β-HCAs are nuclear atypia, an acinar (pseudoglandular) growth pattern, and lack of steatosis.6 β-Catenin and GS immunostaining was used to detect β-HCAs.12,13 Diffuse or focal cytoplasmic GS staining was interpreted as a positive result. Diffuse or focal nuclear β-catenin immunostaining was interpreted as a positive result, whereas cytoplasmic β-catenin immunostaining was recorded as aberrant expression. Characteristic β-HCA histologic characteristics and positive GS and/or β-catenin immunostaining results were indicators of β-HCA.
Inflammatory hepatocellular adenoma
The histologic characteristics of I-HCA are presence of inflammatory infiltrate, marked sinusoidal dilatation, and thick-walled arteries.8 Steatosis may be present in patients with I-HCA but is not as extensive as that in patients with H-HCA.8 Diffuse or focal cytoplasmic immunostaining for SAA was interpreted as a positive result. Typical histologic characteristics and positive SAA immunostaining were indicators of I-HCA.
RESULTS
Clinical information for the eight cases is summarized in Table 1. The male:female ratio was 1:1 and the average age was 28 years. There were three cases of H-HCA, four cases of I-HCA, and one cases of β-HCA. Six cases presented as a single mass. The remaining two cases of multiple masses had underlying glycogen storage disease. One patient with β-HCA had a history of steroid administration for systemic lupus treatment. None of the patients had history of oral contraceptive administration or occurrence of HCC. Morphologic findings (Table 2) and results of immunohistochemical staining (Table 3) were analyzed for each case. None of the cases were interpreted as HCC. This was confirmed by negativity for glypican 3 and p53 immunostaining and a Ki-67 index <1% in all eight cases.
HNF1α-mutated hepatocellular adenoma
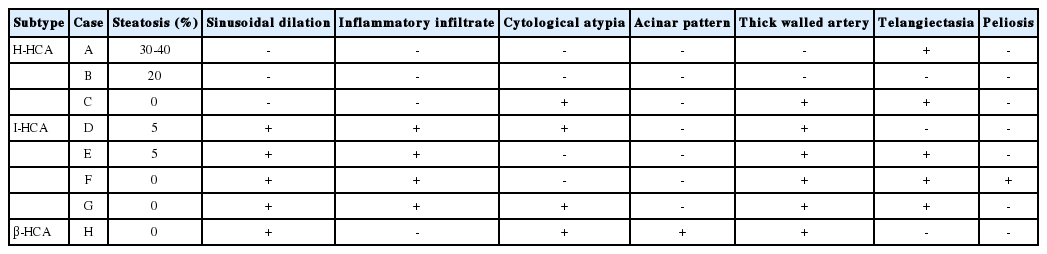
Three cases (A, B, and C) were classified as H-HCA. Two were female and one was male, and one patient had multiple masses. Morphologically, cases A and B revealed marked steatosis of 30-40% and 20%, respectively, and did not show most of the characteristic histologic features of the other subtypes, such as sinusoidal dilatation, inflammatory infiltrate, cytological atypia, acinar pattern, thick-walled arteries, or peliosis (Table 2). Case C did not show steatosis but presented with thick-walled arteries and cytological atypia. All three cases lacked L-FABP expression on immunohistochemical analysis (Table 3). There was faint L-FABP staining in the tumoral area, but it was interpreted as negative because the staining intensity was very weak and identical to the non-specific staining of blood vessels. Based on these findings, these three cases were classified as H-HCA. Cases A (Fig. 1) and B were in accordance with the known characters of H-HCA, but case C was not fully concordant and shared some characteristics with I-HCA.
Inflammatory hepatocellular adenoma
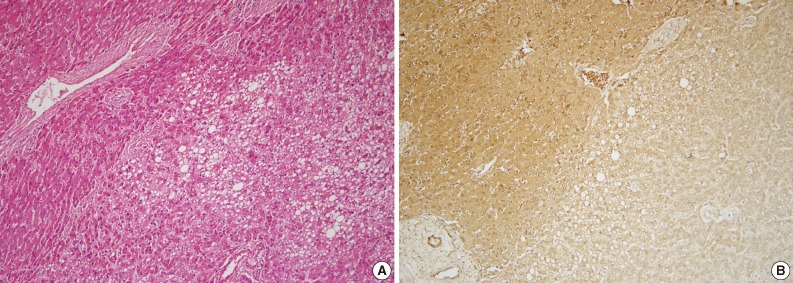
Four cases (D, E, F, and G) were categorized as I-HCA. Three were male and one was female. Cases D, E, and G had an available alcohol history and BMI. Although these three cases had a negative history of alcohol consumption, cases D and G had high BMIs (27.8 kg/m2 and 25.9 kg/m2, respectively). All three cases had morphological characteristics typical of I-HCA, including marked sinusoidal dilation, presence of inflammatory infiltrates, and thick-walled arteries (Table 2). The inflammatory cells were a mixed population of CD3-positive and CD20-positive lymphocytes. In all four cases L-FABP immunostaining was negative and SAA immunostaining was positive (Table 3). Glutamine synthetase immunostaining, should be negative in I-HCA, was positive in cases E and F. Cases D (Fig. 2) and G showed cytological atypia.
β-Catenin-mutated hepatocellular adenoma
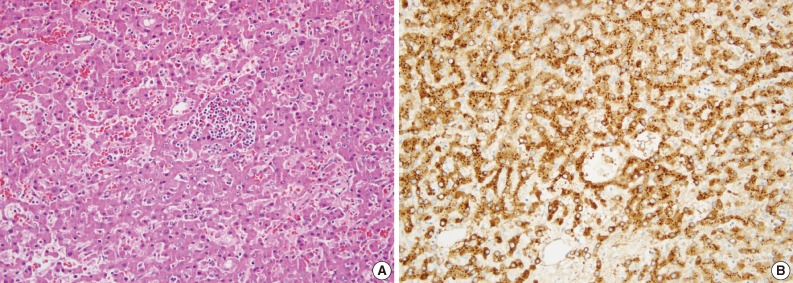
Case H was categorized as β-HCA. This patient had glycogen storage disease. Morphologic analysis showed lack of steatosis and inflammatory infiltrate, but the presence of an acinar pattern with cytological atypia (Table 2, Fig. 3A). By immunohistochemical satining the tumor cells were positive for L-FABP and glutamine synthetase (Fig. 3B). However, β-catenin immunostaining showed an aberrant pattern of diffuse cytoplasmic staining and was interpreted as negative because there was no nuclear staining (Fig. 3C). This case was classified as β-HCA because all morphologic and immunohistochemical findings were in agreement with known characteristics of β-HCA except for the β-catenin immunostaining result. A detailed explanation is given in the Discussion.
Unclassified HCA
All eight cases were determined to belong to the above subtypes therefore none were categorized as an unclassified HCA.
DISCUSSION
This is the largest Korean HCA series review of the past several decades and the first to apply the new subtype classification. The incidence of HCA in Korea is very low and only a few cases of HCA have been reported in Korea during the past 25 years.19 The recent HCA classification was established based on previous studies of genetic and phenotypic characteristics of HCA and hepatic nodular hyperplasia.9,10,15,17,18,20
Pathologically, the subtype classification enables an accurate diagnosis of this rare entity even with a small biopsy specimen.8,21 L-FABP immunohistochemistry is especially helpful in the identification of H-HCA because HNF1α positively regulates FABP1 in normal liver tissue. Mutation in the HNF1α gene causes steatosis,22 therefore most cases of H-HCA also show marked steatosis.7,11 A correlation study between genotype and phenotype of each subtype revealed a strong association between H-HCA and the presence of steatosis, the absence of cytological atypia, and absence of inflammatory infiltrate.6 However, one of our L-FABP-negative cases was not consistent with the description in the new classificat ion system because it did not have steatosis but did have cytological atypia. Although a lack of steatosis in an L-FABP-negative case is unusual, similar L-FABP-negative cases without steatosis can be found in other reports.6,23 According to Zucman-Rossi et al.,6 fewer than 7% of HNF1α-mutated adenomas show no or <10% steatosis and fewer than 2% present with cytological atypia.
Four cases were categorized as I-HCA, and cases showed consistent I-HCA findings including positive SAA and L-FABP immunostaining in addition to an inflammatory infiltrate. Cases D and G showed cytological atypia. Atypia in I-HCA does not seem to be uncommon, and occurred at a rate of approximately 50% in one previous report.6 The Bordeaux group pointed out the presence of cases with overlapping features of I-HCA and β-HCA.13 I-HCAs are have been reported to present with a lower than moderate level of steatosis in 94% of cases.6 In our study, two of the four I-HCAs presented with 5% steatosis while the remaining three showed none.
Case H was categorized as β-HCA because of cytological atypia, an acinar growth pattern, and positive L-FABP staininng. However, this case did not have positive staining for β-catenin. Interpretation of β-catenin immunostaining in HCA seems to be a common problem; thus, the Bordeaux group reported that they rely more on glutamine synthetase immunostaining as a surrogate marker for detecting β-HCA than on β-catenin immunostaining because of the focal distribution or absence of β-catenin positive cells.11 In one Japanese report, the authors also recognized a certain case as β-HCA even if the tumors expressed only GS.24 The discordance between β-catenin mutation status and protein expression in HCAs is not as striking as that reported in studies of other tumors.25,26 Glutamine synthetase, a surrogate marker for β-catenin, showed inconsistent results in our series. Although positive glutamine synthetase staining is supposed to be characteristics of β-HCAs, four of our seven cases with non-β-HCA subtypes showed a positive result. We suggest that the glutamine synthetase staining method does not necessarily indicate β-catenin mutation. Similar findings of inconsistent GS staining results are discussed in a review of HCA cases from Scotland.27 In addition to these difficulties with immunohistochemistry, β-HCA can be difficult or impossible to be distinguished from well differentiated HCC because of the cytological atypia and acinar growth pattern.8
If used alone, none of the histological or immunohistochemical parameters included in the subtype classification seem to be able to properly categorize a particular tumor subtype. As summarized in a table created by the Bordeaux group, a small number of cases from each subtype share histological features with other subtypes.6 Furthermore, immunohistochemistry can give inconsistent results in certain cases. However, L-FABP or SAA staining alone seemed to be sufficient for discriminating between H-HCA and inflammatory HCA, respectively. The opinion that there is no single histologic or immunohistochemical parameter sufficient for classification of any subtype has been expressed in a previous report.27 In our cases presenting with HNF1α-mutated and inflammatory HCA, most, but not all, had intratumoral hemorrhage. Moreover, female predominance is typically observed in HCA cases, but 50% of the patients in our series, were male. A similar relatively high proportion of males was observed in a Japanese study of HCA.24 The authors assumed that this was a characteristic of Japanese HCA cases. When combined with our result, it seems likely that the relatively high proportion of males might be the result of genetic and environmental actors associated with an Asian population. Overall, our results both support and contradict other reports on HCA.
In conclusion, the HCA subtype classification based on genetic and phenotypic characteristics provides new insight into the pathological and radiological diagnosis and clinical management of these tumors. The classification is now generally accepted, but remains controversial. The diagnostic and clinical implications of the new classification scheme may need to be validated by other studies.
Notes
No potential conflict of interest relevant to this article was reported.