A Primary Malignant Rhabdoid Tumor in Adult Liver
Article information
Malignant rhabdoid tumors (MRTs) are rare high-grade malignancies in the renal or extrarenal organs. MRTs of the extrarenal organs mostly affect the liver, central nervous system, pelvis, soft tissue,1 and intra-abdominal cavity.2 Most MRTs occur in infants and young children,3-8 although adult onset MRTs do occur.9 Here, we report a case of primary MRT arising in the liver of a 50-year-old male.
CASE REPORT
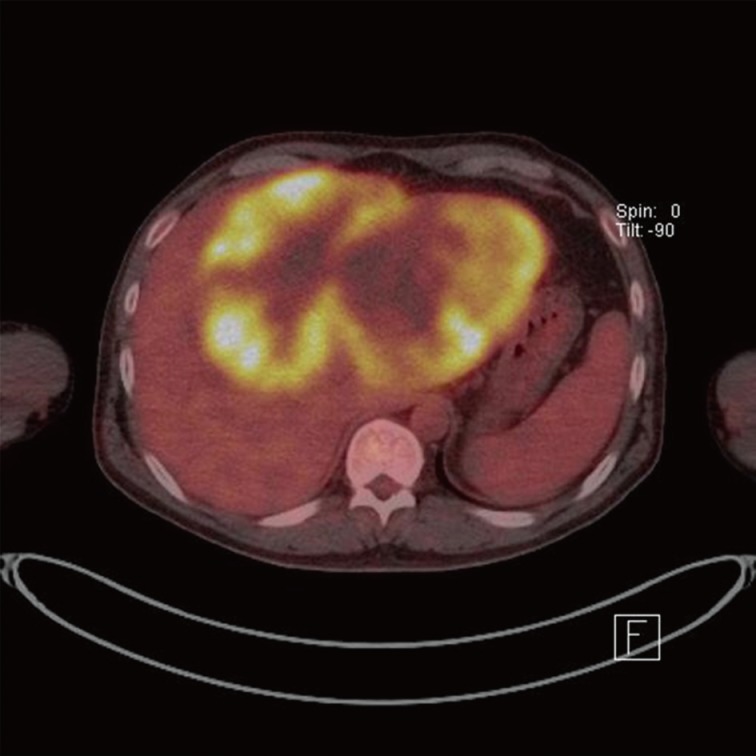
A 50-year-old male had admitted for further evaluation of large hepatic mass. The hepatic mass was found accidently in ultrasonography. His major symptom was a 7-kg weight loss in one month. No gastrointestinal symptoms were present. He had no history of hypertension or diabetes. The serologic markers for hepatitis were negative. His mother had died due to cholangiocarcinoma. On physical examination of the patient, a spider angioma was detected. In the laboratory tests, aspartate aminotransferase/alanine aminotransferase/alkaline phosphatase were increased at 148/54/1,215 U/L, alpha-fetoprotein (AFP) 166 ng/mL, and carcinoembryonic antigen (CEA) 8.19 ng/mL, but cancer antigen 19-9 was in the normal range at 26.6 U/mL. Computerized tomography (CT) showed a very large multinodular hypoattenuating mass with rim enhancement in the whole left lobe and anterior segment of the right lobe (Fig. 1). Also the common hepatic lymph nodes were increased in size. There was no abnormality in each right of left kidney. On positron emission tomography-CT, a large hypermetabolic mass was seen, encompassing the whole left lobe and the anterior segment of the right lobe (Fig. 2).
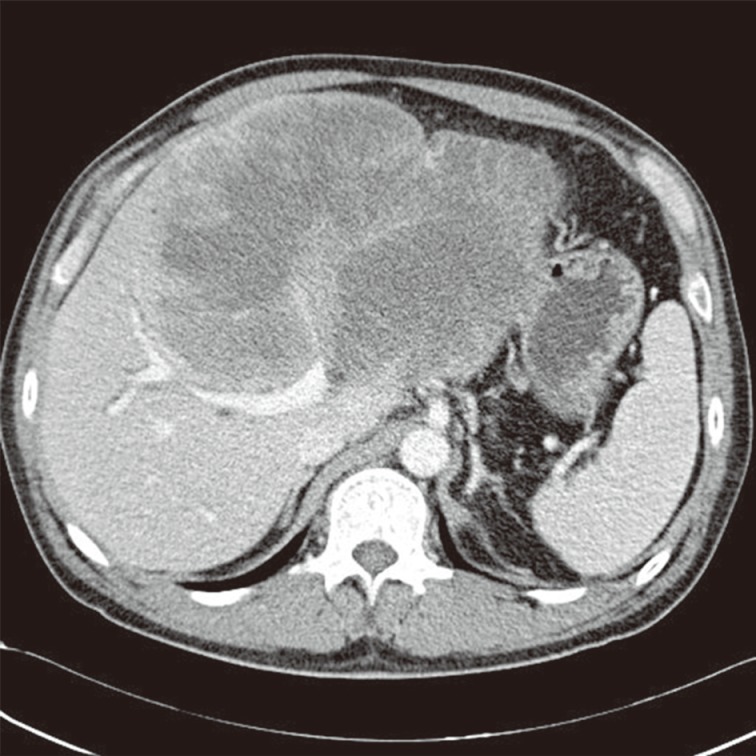
Computed tomography shows a very large hypoattenuating hepatic mass with rim enhancement in the left lobe of the liver.
Pathologic findings
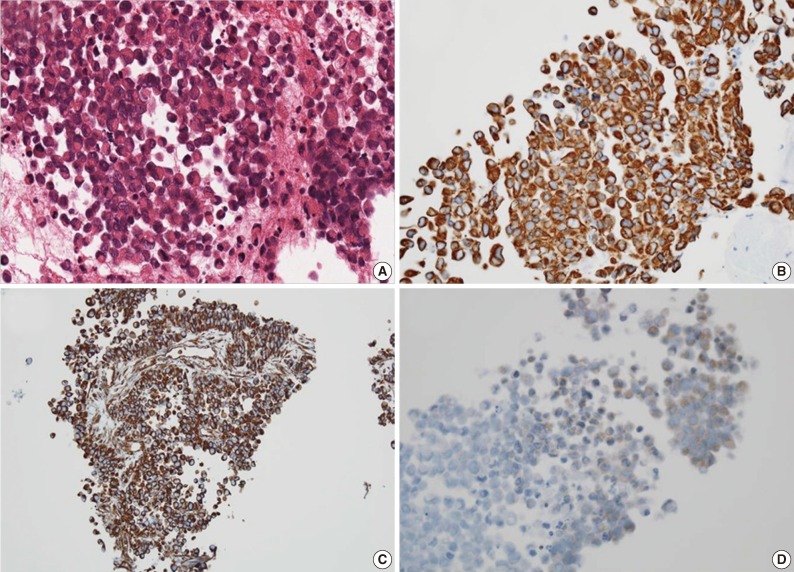
For diagnostic confirmation, CT-guided needle biopsy was performed. The biopsy cores were composed of hypercellular areas alternating with necrosis and hemorrhage. The cellular lesion showed loosely cohesive tumor cell clusters separated by intermediate fibrous septa. The large round tumor cells showed abundant eosinophilic cytoplasm and eccentric vesicular nuclei with prominent nucleoli (Fig. 3A). Abnormal mitoses were frequent. Mucicarmine staining was negative for abundant cytoplasm. The immunohistochemical stains showed diffuse cytoplasmic-positive staining for cytokeratin (CK) 19 and vimentin (Fig. 3B, C) and focal positivity for epithelial membrane antigen and CK7. The immunohistochemical stains for hepatocyte antigen, alpha-fetoprotein, CK20, CD34, CD117, actin, desmin, S100, and human melanoma black 45 (HMB45) showed no reaction in tumor cells. Also the tumor cells showed partial loss of positivity for integrase interactor-1 (INI-1) (Fig. 3D).
DISCUSSION
MRT is a rare aggressive renal or extrarenal tumor arising in infants and young children. Since Beckwith and Palmer10 first described MRT, a few primary or metastatic liver MRTs have been reported in children.1,4-8 Even after that, primary MRT in the adult liver is very rare. Only one MRT in a young adult has been presented in the liver.9
Generally, primary liver MRTs present as a single voluminous liver mass with no other specific clinical symptoms. On serologic tests, AFP and CEA did not increase in most hepatic MRT patients. Due to voluminous hepatic mass formation and no increased tumor markers such as AFP and CEA, primary liver MRTs should be differentiated from hepatoblastoma, hepatocellular carcinoma, and Ewing's tumor in children, and mass-forming cholangiocarcinoma in adults.
Histologically, loosely cohesive tumor cells had abundant eosinophilic cytoplasm and eccentric vesicular nuclei with prominent nucleoli. These features are characteristic in renal/extrarenal rhabdoid tumors. Ultrastructurally, intracytoplasmic filaments were detected. Immunohistochemically, almost all MRTs show dual positivity for CK and vimentin but a negative reaction for alpha-fetoprotein, CD34, CD117, actin, desmin, S100, and HMB45. Loss of INI-1 protein nuclear expression in MRTs is known to be related to a mutation of the hSNF5/INI1 gene. We conclude that the characteristic histology showing a rhabdoid feature and immunohistochemistry indicating loss of INI-1 protein nuclear expression are essential for the diagnosis of MRT. Also, it seems to be significant that this case is the first primary liver MRT arising in an elderly adult among the reports of extrarenal hepatic MRTs.
Notes
No potential conflict of interest relevant to this article was reported.