Primary Squamous Cell Carcinoma of the Upper Genital Tract: Utility of p16INK4a Expression and HPV DNA Status in its Differential Diagnosis from Extended Cervical Squamous Cell Carcinoma
Article information
Abstract
Background
Primary squamous cell carcinoma (SCC) of the upper genital tract, including the endometrium, fallopian tubes, and ovaries, is extremely rare. It must be distinguished from the mucosal extension of primary cervical SCC because determination of the primary tumor site is important for tumor staging. However, patients with SCC of the fallopian tubes or ovarian surface have often undergone prior hysterectomy with inadequate examination of the cervix, making it difficult to determine the primary site.
Methods
We compared histologic findings, p16INK4a expression, and human papillomavirus (HPV) DNA status in four patients with primary SCC of the upper genital tract and five patients with primary cervical SCC extending to the mucosa of the upper genital tract.
Results
All five SCCs of cervical origin showed strong expression of p16INK4a, whereas all four SCCs of the upper genital tract were negative, although one showed weak focal staining. Three of the five cervical SCCs were positive for HPV16 DNA, whereas all four primary SCCs of the upper genital tract were negative for HPV DNA.
Conclusions
Although a thorough histological examination is important, immunonegativity for p16INK4a and negative for HPV DNA may be useful adjuncts in determining primary SCCs of the upper genital tract.
Squamous cell carcinoma (SCC) involving the upper genital tract, including the endometrium, fallopian tubes, and ovarian surface, is extremely rare, with most patients described in single case reports.1-5 Its possible pathogenic mechanisms include 1) de novo carcinogenesis; 2) extensive squamous metaplasia (ichthyosis uteri) in the mucosa of the upper genital tract with subsequent malignant transformation; 3) endometrioid adenocarcinoma with predominantly squamous differentiation; and 4) mucosal spread from cervical SCC.6 Differentiating primary SCC arising in the upper genital tract from primary cervical SCC extending to the upper genital tract is clinically important for tumor staging and patient management, especially since the locoregional recurrence rate is higher and the disease-free survival rate is lower in cervical SCC patients with endometrial involvement than it is in patients without endometrial involvement.7
The diagnostic criteria for primary SCC of the endometrium include the absence of 1) coexisting endometrial adenocarcino adenocarcinoma; 2) a connection between endometrial SCC and the squamous epithelium of the cervix; and 3) a primary squamous lesion in the cervix, either in situ SCC or invasive carcinoma.8 We have found it difficult, however, to determine the primary sites of SCCs detected in a fallopian tube or ovary in patients who have undergone prior hysterectomy with insufficient histological examination of the uterine cervix at the time of surgery.
Human papillomavirus (HPV) infection has been associated with the development of cervical SCC, and p16INK4a, a surrogate marker for HPV infection, is consistently positive in HPV-associated cervical SCCs and precancerous squamous intraepithelial lesions.9 However, the cause of disease and the utility of p16INK4a expression and HPV DNA status have not been clearly determined in patients with primary SCC of the upper genital tract. To determine the utility of p16INK4a expression and HPV DNA status in identifying the primary tumor site, we compared these markers as well as the histologic findings in four patients with primary SCCs of the upper genital tract and in five patients with cervical SCCs extending to the mucosa of the upper genital tract.
MATERIALS AND METHODS
Patient selection
The surgical pathology files of the Department of Pathology of the University of Ulsan Collage of Medicine at the Asan Medical Center in Seoul, Korea, were searched for records of all patients diagnosed between 1999 and 2011 with pure SCCs involving the endometrium, fallopian tubes, and ovaries, regardless of primary tumor site. Patients with SCCs arising in mature teratomas of the ovary, SCCs associated with endocervical-like ovarian mucinous tumors, endometrioid adenocarcinoma with extensive squamous differentiation, and primary cervical SCCs with confluent invasion into the uterine corpus, including the myometrium and endometrium, were excluded. To diagnose primary SCC of the upper genital tract, the entire uterine cervix and endometrium were examined histologically to avoid any failure to identify any minor glandular component of an endometrioid adenocarcinoma, which would lead to its erroneous interpretation as a primary SCC of the endometrium. The records of nine patients with pure SCCs involving the endometrium, fallopian tubes, and/or ovaries were retrieved.
Histologic findings in all nine patients were reviewed by three pathologists (S.H.Y., C.O.S., and K.-R.K.). Based on the presence or absence of in situ or invasive cervical SCC, these patients were classified into two groups. One group consisted of four patients with primary SCC of the upper genital tract, including the endometrium, fallopian tubes, and ovaries, and the second group consisted of five patients with primary cervical SCC and upward mucosal extension. The diagnosis of primary SCC of the upper genital tract was based on a thorough examination of the uterine cervix, and all patients underwent computerized tomography, magnetic resonance imaging, and/or fluorine-18 fluorodeoxyglucose positron emission tomography scanning before or after surgery to detect other possible primary sites.
Clinical information on all nine patients, including age, treatment modality, and follow-up results, was obtained from their medical records.
Immunohistochemical staining
Formalin-fixed, paraffin-embedded tissue sections of all included patients were immunohistochemically stained using a Benchmark automatic immunostaining device (Ventana Medical System, Tucson, AZ, USA), as described previously.10 The samples were incubated with antibodies to p16INK4a (prediluted, CINtecR histology, mtm Laboratories AG, Heidelberg, Germany) and p53 (1:250, Epitomics, Burlingame, CA, USA). The sections were subsequently incubated with biotinylated anti-mouse immunoglobulins, peroxidase-labeled streptavidin (LSAB kit, Dako, Glöstrup, Denmark), and 3,30-diaminobenzidine. Negative control samples omitted the primary antibody. Cervical mucosal samples from patients with high-grade cervical intraepithelial neoplasia were used as positive controls for p16INK4a, while samples from patients with high-grade ovarian serous carcinoma served as positive controls for p53. Slides were counterstained with Harris hematoxylin. Only nuclear staining, with or without cytoplasmic staining, was interpreted as positive for p16INK4a. Expression of p16INK4a was assessed as negative (no expression at all), weak (<30%), moderate (30-90%), or strong (≥90%). Expression of p53 in <10% of cells was classified as negative and expression in >10% was determined to be positive.
HPV genotyping by HPV DNA chip
HPV was detected and genotyped in paraffin-embedded tissue sections from all nine patients. For patients nos. 5-8, who showed tumor involvement of multiple organs, the uterine cervix, and the endometrium, and for patient no. 9, who exhibited tumor involvement of the uterine cervix, fallopian tubes, and ovary, each lesion was separately genotyped.
The HPV genotype was assessed using HPV DNA chips (GoodGene Co., Seoul, Korea), a polymerase chain reaction (PCR)-based DNA microarray system designed to detect 40 subtypes of HPV, including 21 high-risk (HPV 16, 18, 26, 31, 33, 35, 39, 45, 51, 52, 53, 56, 58, 59, 66, 67, 68, 69, 70, 73, and 82) and 19 low-risk (HPV 6, 11, 30, 32, 34, 40, 41, 42, 43, 44, 54, 55, 61, 62, 72, 81, 83, 84, and 90) types.
DNA was extracted from paraffin-embedded tissues using a LaboPass Tissue Mini DNA Purification Kit (Cosmo Genetech, Seoul, Korea). Three sections from each sample were cut to a 20-µm thickness and placed in microcentrifuge tubes. Two sections from each tissue sample were mixed with 1.2 mL xylenes, and the excess xylene was removed with two washes of 1.2 mL 100% ethanol. The dried tissue samples were incubated at 56°C for 30 minutes with a lysis buffer and proteinase K. Each mixture was applied to a spin column and centrifuged into a collection tube, as described by the manufacturer. Purified DNA samples were PCR-amplified in a C1000 Thermal Cycler (Bio-Rad Laboratories Inc., Hercules, CA, USA) with primer sets targeting the L1 and L2 regions of HPV DNA. As a loading control, an ACTB gene sequence from the HEK293 cell line was amplified. PCR products were electrophoresed on 2% agarose gels, with 185 bp products indicative of HPV DNA. For genotyping, 10 µL of each amplified product was denaturated for 3 minutes at 95°C, mixed with a hybridization solution (GoodGene. Co.), applied to the DNA chip, and incubated at 50°C for 30 minutes. The chips were washed twice and dried at room temperature, with hybridized HPV DNA visualized using a DNA chip scanner (Nanostorage, Seoul, Korea). To avoid contamination that may yield false-positive results, all PCR-related work was performed in specialized zones within a PCR laboratory.
Statistical analysis
The difference in mean age between the two groups was assessed using the Mann-Whitney U-test. A p-value <.05 was considered statistically significant.
RESULTS
Clinical findings
Patients with primary SCC in the upper genital tract ranged in age from 34 to 75 years (mean, 53 years), whereas those with primary cervical SCC were between 42 and 79 years of age (mean, 63 years), a difference that was not statistically significant (p=.221).
Of the five patients with primary cervical SCC, two presented with vaginal spotting and one with an abnormal cervicovaginal smear. In one patient, the tumor was incidentally identified after surgery for uterine prolapse, and the tumor in another patient was discovered due to the sudden onset of abdominal pain, nausea, and vomiting during follow-up for cervical SCC in situ. Of the four patients with primary SCC of the upper genital tract, one presented with an intermittent cough, which indicated metastasis to the lung; one each presented with right lower quadrant pain and an ovarian mass, indicative of metastatic symptoms to the ovaries; and one had an incidentally identified endometrial tumor after surgery for a uterine leiomyoma.
Tissue samples were obtained during simple hysterectomy (n=2), modified radical hysterectomy with bilateral salpingo-oophorectomy (n=6), and bilateral salpingo-oophrectomy (n=1). The latter patient had undergone a hysterectomy 10 years earlier at another hospital for a tumor diagnosed as SCC in situ of the uterine cervix. Endometrial extension of the tumor was not recorded at that time, but histologic examination of the endometrium was inadequate. This patient had recurrent tumors in both the fallopian tubes and on the ovarian surfaces.
The tumors in all four patients with primary SCCs of the upper genital tract were diagnosed after thorough examination of the specimens of the uterine cervix obtained at total or radical hysterectomy.
Follow-up duration ranged from 4 months to 13 years (mean, 49.9 months), during which two patients with primary cervical SCC (one each with International Federation of Gynecology and Obstetrics [FIGO] stages IIA2 and IB1) and two with primary SCCs of the upper genital tract died due to the disease. The other five patients were followed up for 13 months to 13.2 years (mean, 75.2 months); one patient with SCC of ovarian origin remained alive with a metastatic tumor to the lung, and the remaining patients were alive without evidence of tumor. The clinicopathologic features of these nine patients are summarized in Table 1.
Histopathologic findings
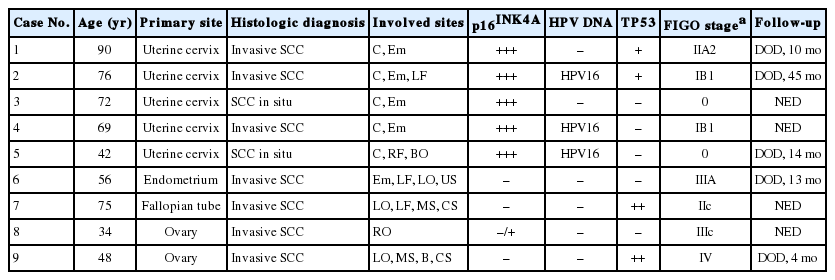
In one patient with primary SCC of the upper genital tract (patient no. 1), the endometrial surface epithelium was partially replaced by mature squamous epithelium with normal endometrial glands underneath (Fig. 1A, B). The polarity of the squamous epithelium was well preserved, and there was little or no cytologic atypia. The remaining endometrium was thin and atrophic, showing chronic endometritis with infiltration of moderate numbers of lymphocytes and plasma cells. The lumens of both fallopian tubes were nearly obliterated, replaced throughout their entire length by metaplastic squamous epithelium (Fig. 1C). Multifocal areas showing invasive growth into the tubal wall were also observed (Fig. 1C, D). The left ovarian surface, the adjacent uterine serosa, and the outer portion of the myometrium were extensively infiltrated by SCC (Fig. 1A, open arrow). Despite obvious stromal invasion into the tubal wall, ovarian stroma, and adjacent uterine serosa, the tumor cells showed only mild nuclear atypia, low mitotic rates (1-2/10 high power field), vesicular chromatin, prominent nucleoli, and abundant clear to eosinophilic cytoplasm (Fig. 1D). Extensive keratin pearl had formed on the uterine serosa and in the outer portion of the myometrium. The pelvic and para-aortic lymph nodes and the omentum were not involved. Histologic examination of the entire cervical mucosa showed no evidence of an abnormal squamous lesion. Endometriosis was not observed in any portion of this patient's tissue samples.

A primary squamous cell carcinoma arising in the endometrium and showing continuous upward mucosal extension and serosal involvement. Squamous lesions in the endometrium (A, solid arrow and B) and fallopian tubes (C, D) show bland cytomorphologic features mimicking squamous metaplasia, with obvious stromal invasion of the tubal wall (C, D), ovary, and uterine serosa (A, open arrow). Note that three samples are completely immunonegative for p16INK4a (E) and one had weak, focal staining (F), which can be interpreted as negative.
The SCC in patient no. 2 was mainly located in the mucosae of the left fallopian tube, extending to the surface of the left ovaovary, mesosalpinx, and colonic serosa. The tumor in patient no. 3 showed involvement of the ovarian capsule, while the tumor in patient no. 4 demonstrated involvement of the pelvic wall, including the mesosalpinx, urinary bladder, and rectal serosa. The tumors in patients nos. 2, 3, and 4 were composed of polygonal squamous cells with pleomorphic nuclei and high mitotic figures, which showed keratinization and intercellular bridges.
Five patients had SCCs of cervical origin, with the tumors extending to the endometrium in patients nos. 5, 7, and 8, to the endometrium and unilateral tubal mucosa in patient no. 6, and to the unilateral tubal mucosa, bilateral ovarian surfaces, and superficial ovarian cortex in patient no. 9. In patient no. 7, the tumor showed continuous extension to the endometrium as SCC in situ without destruction of the basement membrane, whereas in the other four patients, the tumors at multiple sites were not continuous, with multifocal interventions of normal epithelium. The tumors of the uterine cervix were invasive SCC in patients nos. 5, 6, and 8 and SCC in situ in patients nos. 7 and 9 (Fig. 2A). The depths of stromal invasion ranged from 3 to 16 mm. In patient no. 5, the tumor invaded only the superficial stroma of the uterine cervix, as confirmed by examination of the hysterectomy specimens, whereas in patients nos. 6 and 8, tumor invasion was observed in over one-half of the full thickness of the cervix. In the endometrium (Fig. 2B) and fallopian tubes (Fig. 2C), normal epithelia overlay the tumor cells, suggesting their pagetoid spread (Fig. 2D). Finally, patient no. 9, who had undergone a hysterectomy 10 years earlier at another hospital for SCC in situ, underwent surgery for the recurrence of a tumor extending to the mucosa of the bilateral fallopian tubes and bilateral ovarian surfaces (Fig. 2E).
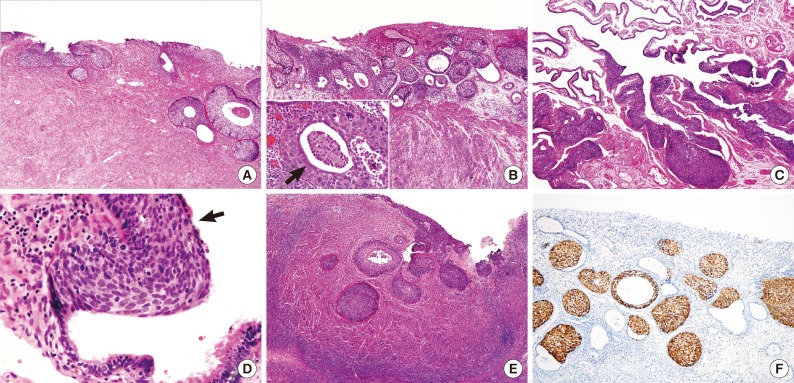
Squamous cell carcinoma in situ with glandular extension of the uterine cervix (A), showing upward extension to the endometrium (B), fallopian tube (C, D), and ovarian surface (E). Note the residual endometrial glandular epithelium (B, arrow) and ciliated tubal epithelium (D, arrow) over the tumor cells. All tumor cells of cervical origin show diffuse strong positivity for p16 (F).
Immunohistochemical expression of p16INK4a and p53 and HPV genotyping
The expression of p16INK4a differed significantly in patients with primary SCCs of cervical origin and those with SCCs originating in the upper genital tract. The primary SCCs in the endometrium and two of the tumors showing involvement of the fallopian tubes and ovaries were immunonegative for p16INK4a (Fig. 1E), whereas one tumor involving the ovarian surface was weakly positive (<10%) (Fig. 1F). All four tumors arising in the upper genital tract were negative for HPV DNA. In contrast, all five tumors of primary cervical origin were strongly positive for p16INK4a, regardless of the involved site (Fig. 2F). Three of these tumors were positive for HPV type 16 (Fig. 3A, B), whereas the other two were negative for HPV DNA.
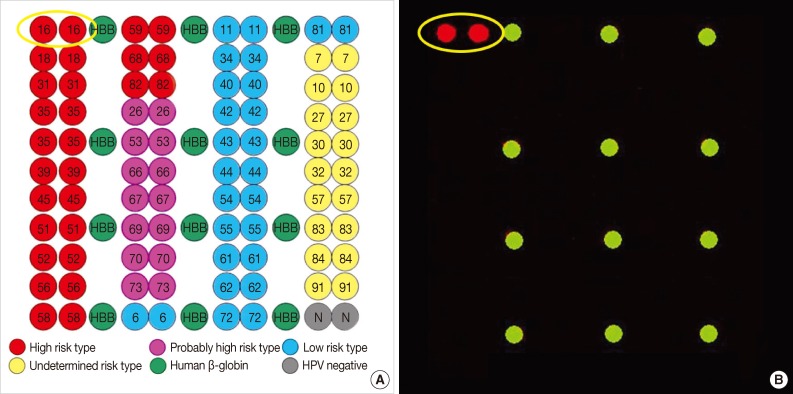
Human papillomavirus (HPV) DNA chip analysis of 40 subtypes of HPV, including 21 high-risk and 19 low-risk subtypes, in the nine patients of this study (A). Three cervical squamous cell carcinomas are positive for HPV DNA type 16 (B).
Immunopositivity for p53 was not consistent in the two groups; its expression was higher in two SCCs of the ovary (30-40%) than in the primary cervical SCCs (1-10%) and the primary SCC of the endometrium (<1%).
DISCUSSION
Although the pathogenic mechanisms of SCCs originating from the uterine cervix and the upper genital tract appear different, both tumor types have the potential for upward mucosal extension to the fallopian tubes, ovarian surfaces, and pelvic cavity, making it difficult to determine the primary tumor site solely by histologic examination or spreading pattern.4,11-13 Moreover, although HPV infection is associated with both squamous intraepithelial lesions and SCCs of the uterine cervix,9 the causes of primary SCCs of the upper genital tract, including the endometrium, fallopian tubes, and ovaries, remain unknown. Endometrial SCCs may originate from heterotopic cervical epithelium within the endometrium14 or from reserve or progenitor cells located between the glandular basement membrane and the endometrial columnar epithelium.15 Hormonal stimulation, pelvic irradiation, vitamin A deficiency, chronic irritation (such as pyometra), chronic endometritis, uterine prolapse or eversion, an intrauterine device, and external irritants may predispose patients to squamous metaplasia and subsequent malignant transformation,13,16-18 but an association with HPV infection has not been documented. By contrast, p16INK4a, a surrogate marker for HPV infection, is consistently positive in HPV-associated cervical carcinomas.9 HPV positivity has been used to identify the primary site in patients with SCC metastatic to pelvic lymph nodes.19
We found that all patients with synchronous or metachromous SCCs in the upper genital tract and uterine cervix had tumors of cervical origin. It was unclear why these tumors of the upper genital tract did not involve the cervix but only extended upward. A discontinuous pagetoid spread of malignant tumors through the mucosa with intervening uninvolved mucosa is a common finding in many organs, including the breast and vulva. We observed a pagetoid spread in cervical SCCs extending to the endometrium and fallopian tube, a finding also noted in non-primary SCCs of the upper genital tract. HPV and p16INK4a have been detected in SCCs of the fallopian tube and cervical SCCs in situ, suggesting lesion multiplicity and a "field effect" of HPV infection.20 However, if HPV infection has a field effect on normal upper genital tract mucosae, the incidence of endometrial SCC should be higher than currently reported. The absence of stromal invasion in cervical squamous lesions of patients with invasive SCC of the upper genital tract or the absence of continuity between SCCs of the uterine cervix and upper genital tract does not necessarily indicate that SCC of the upper genital tract is an independent primary tumor. Although SCC in situ and microinvasive SCC of the uterine cervix with stromal invasion <5 mm rarely metastasize to other organs or lymph nodes, SCC in situ at any site in the uterus has the potential to spread upward within the basement membrane and invade any intervening organ, followed by metastasis.8,21,22 Thus, the deepest focus of invasion may not represent the primary tumor site. Since extensive squamous differentiation can be observed in endometrioid adenocarcinomas of the endometrium and ovary, the failure to sample minor glandular components of endometrioid adenocarcinomas may lead to an erroneous interpretation of the tumor as being a primary SCC of the endometrium or ovary. Meticulous histological examination of the mucosae of the entire upper genital tract in our patients, however, failed to identify a glandular component.
Our results provide further evidence that SCCs of the uterine cervix and upper genital tract have different pathogenic mechanisms. Moreover, the presence of HPV DNA and p16INK4a may indicate that a lesion is of cervical origin. HPV negativity in samples from two of our patients may have been due to the long duration of storage in the paraffin blocks. The association between p53 expression and differentiation of the primary site was not clear.
Primary endometrial SCCs may arise from ichthyosis uteri.18,23,24 A review of 34 patients with primary SCC of the endometrium showed squamous metaplasia in 11 (32%),25 suggesting that squamous metaplasia is an important predisposing condition to SCC of the endometrium. One of our patients (patient no. 1) had continuous squamous lesions from the endometrium through the fallopian tubes to the ovarian surface, uterine serosa, and pelvic cavity. Cytologically, however, these tumor cells were indistinguishable from those of squamous metaplasia, but obvious stromal invasion indicated a malignant tumor. Thus cytologic atypia of the squamous epithelium alone is not an absolute criterion for the diagnosis of endometrial SCC. Rather, squamous metaplasia may be a preneoplastic or neoplastic lesion.
Most primary SCCs in the ovaries derive from the malignant transformation of squamous epithelium in mature cystic teratomas. Rarely, however, these tumors may arise from endometriosis of the ovary26-28 or in association with a Brenner tumor or endocervical-like mucinous tumor of the ovary.29 Primary SCC of the ovary without any underlying lesion is extremely rare, with fewer than 30 cases described in the English language literature.30 Tumors at the ovarian surface may have extended from the endometrium or fallopian tube, as in patient no. 9 in this study.6 Therefore, meticulous histological examination of the entire endometrium and fallopian tubes is needed to determine the primary tumor site and to trace the route of tumor spread.
We observed no significant differences in the overall clinical outcome between the two groups, although the number of patients examined was small. The patient with primary SCC of the endometrium with little or no cytologic atypia died of the disease, suggesting that the presence or absence of cytologic atypia was not closely correlated with patient prognosis.
In conclusion, although thorough histologic examination is needed to determine whether an SCC of the upper genital tract is a primary tumor or an extension from the cervix, assessment of p16INK4a expression and HPV DNA status can be useful adjuncts in hard-to-diagnose tumors.
Notes
No potential conflict of interest relevant to this article was reported.