Diagnostic Accuracy of Cerebrospinal Fluid (CSF) Cytology in Metastatic Tumors: An Analysis of Consecutive CSF Samples
Article information
Abstract
Background
Cerebrospinal fluid (CSF) examination can be used to verify the presence of primary malignancies as well as cases of central nervous system (CNS) metastasis. Because of its importance, there have been several studies concerning the sensitivity of CSF cytology. To determine the practical use and reproducibility of diagnoses based on CSF cytology, we evaluated this test by analyzing cytology results from consecutive CSF samples.
Methods
Between July 2010 and June 2013, 385 CSF cytology samples from 42 patients were collected. The samples were gathered using a ventricular catheter and reservoir. CSF cytology of all patients was examined more than two times with immunocytochemistry for cytokeratin.
Results
Primary neoplastic sites and histologic types of patients' metastatic cancer were diverse. The overall sensitivity for detecting malignancy was 41.3%. Even within short-term intervals, diagnoses frequently changed.
Conclusions
Our results were inconsistent, with low sensitivity, when compared to the results of previous studies. However, CSF evaluation can still provide valuable diagnostic and prognostic information because adjuvant treatments are now routinely performed in patients with CNS metastasis. Negative CSF cytology results should not be ignored, and continuous CSF follow-up is essential for following the clinical course of patients with metastatic cancer involving the CNS.
Since the first evidence of malignancy in cerebrospinal fluid (CSF) was reported in 1904, CSF examination has been used to diagnose numerous neoplastic diseases of the central nervous system (CNS).1 In patients with CNS metastasis, diagnostic confirmation is provided mostly through CSF cytology and serves to verify the presence of malignancy.2,3 As most secondary CNS tumors spread along the leptomeningeal space and communicate with the ventricles or subarachnoid space, it is generally not difficult to detect malignant cells in the CSF. Several studies concerning the diagnostic accuracy of CSF cytology have been reported.4-6 In those previous studies, however, CSF collection and examination were performed only once per patient, and the results varied greatly depending on the sampling time. Moreover, it is difficult to judge patient status using a single cytological examination because continuous adjuvant therapies are routinely performed.7 To determine the practical use and reproducibility of diagnoses based on CSF cytology, we evaluated this test by analyzing cytology results from consecutive CSF samples.
MATERIALS AND METHODS
Patient selection
Between July 2010 and June 2013, 385 CSF cytology samples were collected from 42 patients with the presence of a metastatic tumor confirmed by at least two histologic or cytological studies. Cytology samples obtained before adjuvant therapies were excluded to evaluate the diagnostic rate of CSF cytology more consistently. The breakdown of the patient population was as follows: 25 males and 17 females, with a median age of 55 years (range, 29 to 77 years). The mean observation period was 5 months (range, 1 to 22 months), and the mean number of CSF examinations was 9 (range, 2 to 34).
Cerebrospinal fluid specimen collection and cytology slide preparation
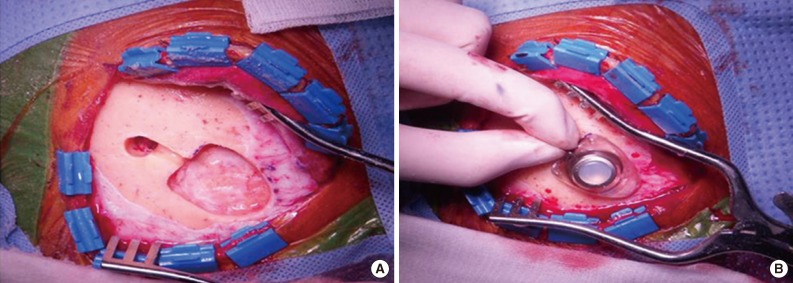
All patients underwent an operation for the placement of a ventricular catheter and reservoir as well as consecutive CSF collections using the reservoir (Fig. 1). To minimize dry artifacts and prevent cell degeneration, samples were delivered to the Department of Pathology immediately upon collection. Samples were then processed using liquid-based cytology (LBC) (ThinPrep, Cytyc Co., Boxborough, MA, USA), an automated method of preparation and smearing of cells in a monolayer. Slides were stained and evaluated with the Papanicolaou staining method. Of the 385 CSF samples, 54 were processed by a conventional smear method rather than the liquid-based method because those samples were obtained when the liquid-based method was not available for CSF cytology.
Immunocytochemistry
Immunocytochemistry (ICC) was performed using a Ventana XT automated stainer (Ventana Co., Tucson, AZ, USA) with an antibody to cytokeratin (1:300, AE1/AE3, Dako, Carpinteria, CA, USA). Slides were incubated with primary antibody for 32 minutes at 37℃ followed by a universal secondary antibody for 8 minutes at 37℃. Slides were incubated in streptavidin-horseradish peroxidase D for 16 minutes at 37℃, and then the substrate, 3,3'-diaminobenzidine tetrahydrochloride (DAB) H2O2, was added for 8 minutes, followed by hematoxylin and bluing reagent counterstains at 37℃.
Interpretation criteria
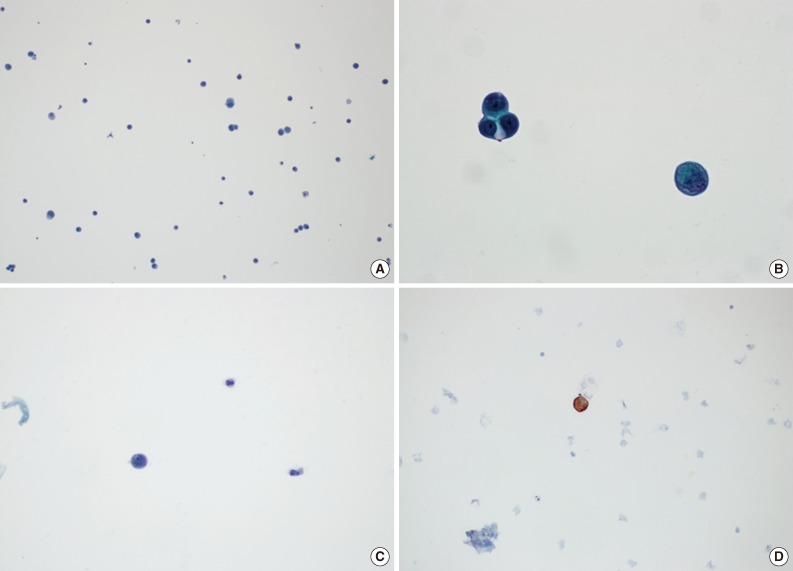
Cases without atypical cells suggestive of metastasis were diagnosed as negative for malignancy. A positive diagnosis of malignancy was defined as the presence of atypical cells with cytokeratin immunoreactivity, regardless of the amount (Fig. 2). There were some cases that presented with atypical cells on Papanicolaou-stained slides, but it was not possible to evaluate many of these cases using cytokeratin ICC slides because the cells disappeared during the staining process. In those cases, we termed the diagnosis suspicious for malignancy, and these cases were regarded as positive findings when calculating the diagnostic rates. All slides, including those for ICC, were independently reviewed by two pathologists (S.H.K and Y.S.B).
RESULTS
As summarized in Table 1, primary malignancy sites were diverse. Lung was the most common primary site with 22 cases, followed by breast with 6 cases. The histologic types of primary malignancies are shown in Table 1. Of the 385 specimens, 132 were diagnosed as positive for malignancy and 27 were suspicious for malignancy, for a total of 159 specimens considered to have a positive diagnosis of malignancy. Among the 42 patients, 3 were never diagnosed as malignant by cytology examination even though all were confirmed to have CNS metastasis on brain tissue biopsy. Eight patients were consistently diagnosed as positive for malignancy in serial CSF evaluations. The positive malignancy diagnosis rates in the other 31 patients ranged from 4.5% to 87.5%, and the mean positive rate was 41.3%.
Table 2 demonstrates two representative cases (case nos. 4 and 12) from our series. During a period of two months, these two patients underwent 13 and 15 CSF cytology examinations, which yielded positive results in 6 and 10 of the tests, respectively. The intervals between CSF cytology examinations ranged from 0 to 13 days and were short enough to enable more meticulous analysis of consecutive diagnostic rates. However, the results appeared random, without a consistent trend. Even within the same day, the diagnoses made from two specimens were different.
DISCUSSION
Rapid transport is important for optimal cellular preservation in CSF materials, which can be cytolysed quickly. In order to reduce the number of nondiagnostic cases, CSF materials should be examined as soon as possible after collection. From a routine diagnostic viewpoint, degeneration is one of the most problematic artifacts when diagnosing cytology slides, as CSF specimens tend to degenerate more readily than other cytology specimens. These artifacts can affect the diagnostic accuracy and reduce specimen adequacy. We were able to control artificial factors by reminding clinicians of the importance of rapid processing and encouraging them to submit the samples immediately.
Most previous studies concerning the diagnostic rates of CSF examination used the lumbar puncture as a diagnosing modality.4-6 However, lumbar puncture can be harmful to patients and difficult for clinicians because it is an invasive procedure. It is this for reason that most previous studies regarding the diagnostic accuracy of CSF performed only one CSF examination per patient. In this study, however, consecutive CSF sampling was performed using the ventricular catheter and reservoir from each patient. Although a neurosurgical procedure is required to implant a ventricular catheter in patients, it enables the continuous collection of CSF and consecutive analysis.
LBC is now a widely used method for preparing cytology samples and has achieved broad acceptance for most cytology specimens.8-11 Furthermore, for the diagnosis of metastatic tumors in CSF, thin-layer LBC has been suggested as an appropriate diagnostic method.12,13 As in immunohistochemistry, ICC improves the diagnostic rates because cytology is often difficult and problematic to evaluate using cellular morphology alone.14 Using LBC is more convenient for performing ICC than conventional smear techniques, and the diagnostic efficacy of ICC on smears processed by thin-layer LBC has been previously validated.15-17
Up to the present time, there have been several reports regarding the diagnostic accuracy of CSF cytology in patients with CNS metastasis. Wasserstrom et al.5 reported a sensitivity of 54.4% in initial examination and 91.1% in subsequent examination. Gondos and King6 also reported a sensitivity of 53.3%. Even though we used implanted ventricular catheters when obtaining CSF and LBC when preparing CSF slides, results of our study showed much lower diagnostic rates than previous studies. The discrepancy between results of previous studies and ours might be due to the different process of gathering CSF; unlike previous studies, we tried to evaluate the diagnostic rates of CSF cytology using consecutive examination of CSF samples from each patient. Moreover, in our study, samples were obtained from patients receiving adjuvant treatments such as chemotherapy and radiation therapy, which may have affected the diagnostic rates of CSF analysis. From the viewpoint of daily practice, our results may be more practical and accurate because most patients with CNS metastasis are now managed with ancillary treatments.7
We could not analyze the results of CSF examinations with statistics of sensitivity or specificity because not all cytology specimens had a concordant tissue biopsy. In other words, the negative findings could not be directly considered as false negatives because we could not completely exclude the possibility that the negative findings resulted from true tumor regression due to adjuvant therapies. However, as shown in Table 2, results were distributed unevenly although all examinations were performed within very short intervals, even within the same day in some cases. Therefore, we suggest that the negative findings are not the results of tumor regression but false negative results. If so, the value of 41.3% can be regarded as sensitivity of CSF cytology in patients being treated with adjuvant therapies due to CNS metastasis.
Several possible explanations exist for the negative results. First, several factors influencing the quality of CSF cytology specimens should be investigated. In our study, cytology specimens from positive cases were more cellular than those from negative cases. However, this cannot be the reason for the negative results because CSF is physiologically acellular. The volume of the submitted specimen can affect the diagnostic accuracy, but unfortunately this aspect was not evaluable because the amount of fluid was not promptly recorded. Dry artifact is one of the most important factors in determining the quality of CSF slides. As mentioned earlier, we could maintain the quality of CSF cytology by notifying clinicians of the importance of rapid transport. However, specimen transport could not be controlled precisely and evenly because the specimens were transported by different clinicians with different time intervals. There was no significant difference among negative results when grouped according to the sites of primary tumors (data not shown). Taken together, the negative results should be regarded as a multifactorial phenomenon without a specific reason.
Temporary CSF cytology examinations are no longer sufficient for estimating clinical course because chemotherapy and optional radiotherapy are generally standard treatment modalities in patients with CNS metastasis. Although the total number of cases included was not remarkable, this study is noteworthy in that we tried to evaluate the diagnostic rates of CSF cytology in a novel way. By analyzing consecutive CSF samples obtained within short-term intervals, we were able to evaluate the diagnostic rates of CSF examination more precisely and more practically. Although the sensitivity of CSF cytology as a diagnostic tool in patients with CNS metastasis was lower than expected, even with the ICC, we consider this result to be important and instructive as negative results should not be ignored or regarded as tumor regression. Alternatively, continuous follow-up using CSF cytology is essential for determining clinical course, and the results must be interpreted in conjunction with clinical and radiological findings.
Acknowledgments
This study was supported by a faculty research grants from Yonsei University College of Medicine for 2013 (6-2013-0027) and the Basic Science Research Program through the National Research Foundation of Korea, funded by the Ministry of Education, Science and Technology (2010-0021092) for Dr. Se Hoon Kim.
Notes
No potential conflict of interest relevant to this article was reported.