Diffuse Large B-Cell Lymphoma Arising in Warthin's Tumor: Case Study and Review of the Literature
Article information
Abstract
Warthin's tumor is the second most common type of salivary gland tumor. Microscopically, Warthin's tumor displays a proliferative epithelial component and lymphoid stroma. Carcinomas arising from the epithelial component are well known, but malignant transformations of the lymphoid stroma are rare. When they do occur, they are most commonly B-cell type non-Hodgkin lymphomas. A 60-year-old male patient underwent surgical resection of a parotid mass. After superficial parotidectomy, microscopic examination indicated that the tumor was of epithelial components with basaloid and oncocytic columns of cells neighboring lymphoid components. In addition to the lymphoid follicles with distinct germinal centers, there were large, bizarre and extremely atypical neoplastic cells seen in the lymphoid component. Large neoplastic cells were diffusely CD20 and CD30 positive. The patient was diagnosed with "Warthin's tumor and diffuse large B-cell lymphoma with expression of CD30." The histopathologic and clinical features are discussed along with a review of the literature.
Warthin's tumor (WT) is a benign neoplasm with a proliferative epithelial component of the salivary gland epithelium and the lymphoid stroma.1 It is commonly found in the lower pole of the parotid gland, and an estimated 12-20% are multicentric and 5-14% are bilateral.2-4 Approximately 1% of WT undergo malignant transformation, which can be seen in both the epithelial and lymphoid components.5 While some studies indicate a higher frequency of malignant transformation in the lymphoid components, others indicate that non-Hodgkin's lymphoma diagnosed in WT excision materials is almost rare.1,4 Furthermore, literature indicates that some malignant lymphoma cases are a component of disseminated disease in WT excision.6 This paper presents a diffuse large B-cell lymphoma (DLBCL) in WT, found in superficial parotidectomy material.
CASE REPORT
A 60-year-old male patient was referred to an otorhinolaryngology clinic due to a lump on the left side of his jaw, which had grown in 2 months. Ultrasound sonography test examination revealed a cystic mass that was 24×17 mm in size with smooth contours. Multiple echogenic and reactive lymph nodes with partially visible hila were visualized in the neighboring upper jugular chain, with the largest being 16×10 mm in size. Following a neck magnetic resonance imaging and a preliminary diagnosis of WT, a left superficial parotidectomy was conducted. The patient had a history of smoking, hypertension, and coronary artery disease.
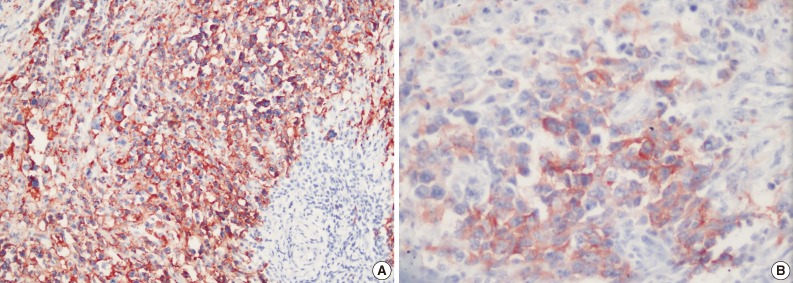
Left superficial parotidectomy materials were sent for pathologic examination in two pieces, which were 5×3.2×2 cm and 4.5×3×1.2 cm in size. Cross section analysis showed an off white-yellowish, well-contoured nodular tumor with a bleeding center of 4×2.5×2.2 cm. Microscopic examination indicated that the tumor had epithelial components with basaloid and oncocytic columns of cells neighboring lymphoid components (Fig. 1). In addition to the lymphoid follicles with distinct germinal centers, infiltration of large neoplastic cells with bizarre and extremely atypical morphology was seen in the lymphoid component (Figs. 2, 3). The large neoplastic lymphoid cells constituted more than 50% percent of the lymphoid component.
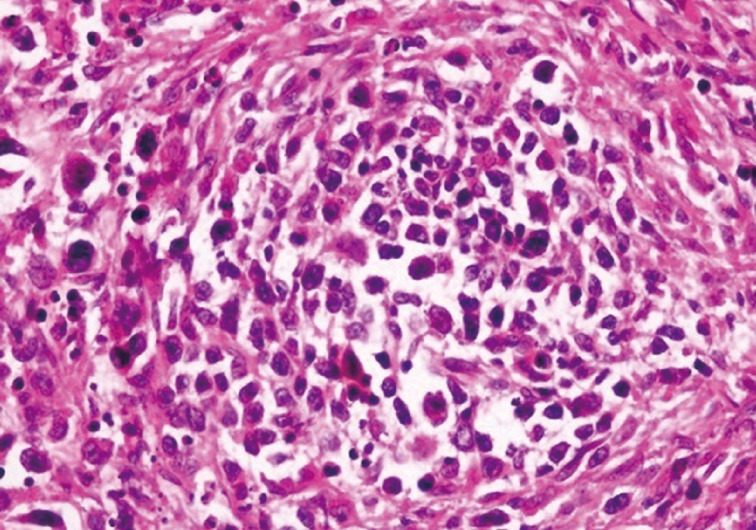
Neoplastic lymphoid cells display scanty cytoplasm, large nucleolus, and prominent nucleoli. Atypical mitotic figures are also seen.
Immunohistochemical examination showed that neoplastic cells expressed strong positivity for CD20 (Fig. 4A), CD79a, CD30 (Fig. 4B), leukocyte common antigen, IgG, CD138, MUM1, and focal positivity for kappa. Staining for lambda, IgM, IgA, CD3, CD5, CD10, CD15, CD56, epithelial membrane antigen, Bcl2, Bcl6, cyclinD1, S100, pancytokeratin, cytokeratin 20, human melanoma black 45, actin, and desmin were negative. Latent Epstein-Barr virus (EBV) was shown to be negative in tumor cells by using EBV-encoded RNA chromogenic in situ hybridization. Due to these findings, the patient was diagnosed with "WT and CD30 positive diffuse large B-cell lymphoma in the parotid gland."
Following the lymphoma diagnosis, a full body screen was performed. Results indicated lymphadenopathies of a pathologic size in the inguinal and iliac regions. In addition to these findings, the left suprarenal gland showed two nodular mass lesions, which were assessed as likely adenomas; however, this preliminary diagnosis was not confirmed by histopathology. Bone marrow biopsy revealed a normocellular bone marrow with no lymphoma involvement.
The patient was stage 3A and received six courses of rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) therapy. During 6-month follow-up, the patient was free of disease.
DISCUSSION
WT is the second most common type of salivary gland tumor. In 10-15% of cases, it is bilateral, and it accounts for 70% of all bilateral salivary gland tumors.2 The male/female ratio is 1.6/1, and it typically develops in the 6th and 7th decades. Smoking increases the risk of developing WT.5 Microscopically the tumors are typically composed of proliferative epithelial components accompanied by lymphoid stroma with lymphoid follicles that have distinct germinal centers. Histogenesis of the lymphoid stroma in WT has been a topic of discussion for many years.
Lymphoid stroma can arise as a cell response to epithelial neoplasms or as a normal lymph node due to residue held by the epithelial neoplasm.5,6 The most widely accepted hypothesis suggests that WT is a neoplasm that develops in the heterotopic salivary gland ductus within or around the parotid lymph nodes.7 Transformation to carcinoma in WT is a well-known phenomenon; however, the development of lymphomas from WTs is very rare.4,8 Although some cases contain a normal residual lymphoid component, in others cases the lymphoid component contains entirely neoplastic lymphoid cells.4 In the present case, non-neoplastic lymphoid tissue was also present in the neighboring areas.
The pathogenesis of malignant transformation of WT remains unclear; however, exposure to radiation is of particular interest, as the relationship between previous radiotherapy and lymphomas arising from WTs has been determined by some authors.4,5,9
Chronic immune sialadenitis is thought to play an important role, independent of the presence of Sjögren syndrome symptoms.4,7,10 In this case, there was no history of radiotherapy or sialadenitis, but a history of smoking may have provoked the development of WT.
Saxena et al.1 state that because the lymphoid stroma of WT is part of the systemic lymphoid tissue, in patients with lymphomatous spread of WT, disseminated disease is present during the staging either at the time of the diagnosis or after.
In the present case, bone marrow biopsy showed no disease involvement. With screening techniques, lymphadenopathies of a pathologic size were found in the inguinal and iliac regions. This indicates that disseminated disease may have been present synchronously.
Some researchers suggested that although the relationship between WT and lymphoma could be coincidental, it might also be of a pathogenic nature. According to the latter statement, a single agent can affect different tissues or one tumor could trigger the formation of another. From this point of view, the epithelial component is a continuous antigenic stimulator for the lymphoid component, which provides the stimulus for the development of lymphoma.1,6,8 According to this theory, the frequently observed reactive follicular hyperplasia in WT may be histological evidence of chronic antigen stimulation.1
It has been suggested that the lymphomas seen with WT are typically non-Hodgkin lymphomas; however, there are a few cases reporting Hodgkin's lymphomas.11,12 The majority of non-Hodgkin's lymphomas in WT are follicular lymphomas. DLBCL, small lymphocytic lymphoma, extranodal marginal zone lymphoma of mucosa associated lymphoid tissue, and mantle cell lymphoma have also been reported.4,6,8,9 A small number of T-cell lymphomas such as peripheric T-cell lymphoma and T-cell lymphoblastic lymphoma have also been described in WT.4,8,13
In summary, malignant lymphomas in WT are very rare. The presented case is a diffuse large B-cell lymphoma expressing CD30 positivity. To the best of our knowledge this is the first case in literature describing DLBCL with expression of CD30 in WT.
Notes
No potential conflict of interest relevant to this article was reported.