Crush Cytology of Microcystic Meningioma with Extensive Sclerosis
Article information
Microcystic meningioma is an unusual type of meningioma that has been described under various names including humid, myxomatous, vacuolated, or arachnoid trabecular cell meningioma.1 Histologically, microcystic meningiomas are characterized by numerous cystic spaces filled with edematous fluid and intermixed meningothelial cells of stellate or spider shape. Cytological descriptions of microcystic meningioma have been limited.2,3
We recently experienced a case of microcystic meningioma with extensive sclerosis. Intraoperative crush cytologic diagnosis was difficult to establish for the following reasons. First, in microcystic meningiomas, extensive sclerosis is extremely rare. Second, crush cytologic findings of microcystic meningiomas with extensive sclerosis differ from those of conventional meningiomas.
Here, we emphasize the importance of cytologic diagnosis of this uncommonly encountered microcystic meningioma with extensive sclerosis.
CASE REPORT
A 63-year-old female patient presented with a three-month history of dysarthria and motor weakness of the right arm. Enhanced brain T2-weighted magnetic resonance (MR) images showed a mass measuring approximately 5.4 cm in the left frontal convexity with strong enhancement (Fig. 1). Intraoperatively, the subdural mass showed marked adhesion to the underlying brain parenchyma and dura, and the lesion had a large feeding artery and high vascularity. Near total removal was performed.

Enhanced T2-weighted images show a well-demarcated mass (arrow) of high signal intensity as well as low focal signal.
Cytological findings
Intraoperative crush preparations stained with hematoxylineosin showed paucicellular smears composed of a few noncohesive spindle-to-ovoid cells, fragmented collagenous materials, and rare cohesive whorls of round to polygonal eosinophilic cells with a background of bluish mucin (Fig. 2A). Scattered polygonal-to-spindle cells contained abundant vacuolated, clear bubbly cytoplasm (Fig. 2B, left). Rare intracytoplasmic microcysts were observed (Fig. 2B, right). Nuclear hyperchromasia was frequently found. There were a few clusters of tumor cells having indistinct cell borders and lacy eosinophilic cytoplasm, consistent with whorl-like structures (Fig. 2C). Some scattered polygonal cells had vacuolated cytoplasm within a collagenous material (Fig. 2D). In that portion, the tumor cells had eosinophilic cytoplasm with a well-defined border and a refractile, thick hyaline nature, rather than a lacy or fibrillary one (Fig. 2E). There were collagen nodules and calcific materials (Fig. 2F). No nuclear pseudoinclusions were found. Neither mitosis nor necrosis was found.
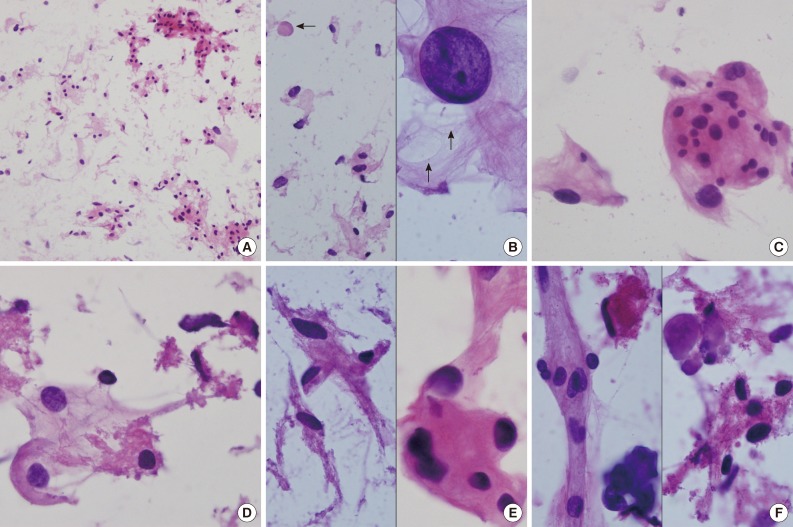
(A) Crush cytology smears show sparse cellularity composed of scattered individual cells and a few clusters of eosinophilic cells in bluish mucinous materials in the background. (B) Left: Scattered polygonal cells have abundant vacuolated bluish cells. Note background bluish mucinous materials and solid hyaline materials (arrow). Right: Hyperchromatic round nuclei and lacy cytoplasm with microcysts (arrows). (C) Meningothelial whorls of eosinophilic cells have indistinct cell borders and occasional nuclear hyperchromasia. (D) The vacuolated cytoplasm also shows collagenous hyalinized cytoplasm. (E) Left: Collagen-deposited cells show low nuclear/cytoplasmic ratio. Right: Note refractile and eosinophilic hyaline cytoplasm with feathering off. (F) Calcified materials and collagens are scattered in background.
Histologic findings
The near totally removed mass was composed of a microcystic area and a markedly sclerotic area occupying approximately 80% of the mass. Scattered stellate, vacuolated cells and foam cells were observed within the intercellular microcystic area (Fig. 3A). Pleomorphic cells with enlarged hyperchromatic nuclei were occasionally found in both sclerosing and microcystic areas. The fibrotic area was composed of fibrous nodules and collagen bundles as well as markedly hyalinized vessels with focal foci of round-to-oval shaped cells in the fibrotic nodule (Fig. 3B). In the fibrotic collagenous portion, some spindle shaped tumor cells were found in collagenous nodules and thick hyalinized vessels (Fig. 3C). Psammoma bodies were frequently noted. Neither mitosis nor necrosis was found. These findings were reminiscent of microcystic meningioma. Both the spindle cells in the fibrotic area and the meningothelial cells in the cellular portion of typical microcystic meningioma demonstrated positivity for vimentin (prediluted, V9, Dako, Glostrup, Denmark) and negativity for glial fibrillary acidic protein (prediluted, polyclonal, Dako) and S-100 protein (prediluted, polyclonal, Dako). Only meningothelial cells showed focal cytoplasmic reactivity for epithelial membrane antigen (prediluted, E29, Dako). Masson-Trichrome staining highlighted the thick fibrotic collagenous changes of the mass. The edematous fluid in the microcysts was not stained with periodic-acid Schiff or mucicarmine, and was weakly positive for Alcian blue and toluidine blue. The tumor was diagnosed as a microcystic meningioma with extensive sclerosis, World Health Organization grade 1.
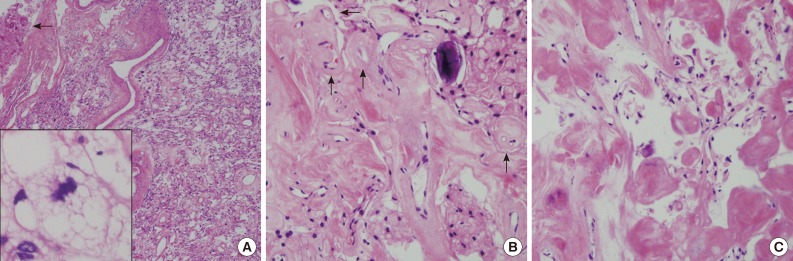
(A) The resected mass is composed of mainly sclerosed and fibrotic collagenous deposits; however, the characteristic portion of microcystic meningioma is also found. Arrow indicates adhered brain parenchyma without tongue-like tumor invasion. Inset indicates stellate, spider-like multivacuolated cells in the microcystic area. (B) In the fibrotic portion, the residual meningioma cells are transformed to massive sclerosis. Arrows indicate hyalinized vessels. (C) High magnification of the sclerotic portion shows some spindle-shaped fibroblast-like cells in abundant eosinophilic, collagenous deposits.
After the operation, the patient demonstrated good recovery.
DISCUSSION
Microcystic meningioma is characterized by multiple cystic spaces and entrapped vacuolated or stellate-shaped tumor cells as well as a rich vasculature.1 The cytoplasm of the tumor cells is more abundant than that of classic meningioma, and have a faintly eosinophilic or cyanophilic vacuolated appearance resembling hemangioblastoma with lipid. These pathologic findings are not discrepant to dense enhancement on MR imaging, low signal intensity in T1-weighted images, and high signal intensity in T2-weighted images with frequent peritumoral edema.4
Intraoperative crush cytology frequently provides helpful clues in diagnosis of central nervous system tumors. However, cytology of meningioma is known to show a great variety of cytologic diversity; the same can be true for histologic diversity. Meningioma of the conventional type is usually easy to recognize cytologically; cohesive syncytial meningothelial whorls, psammoma bodies or the presence of intranuclear inclusions or grooves, and refractile and wispy, rather than fibrillary, cytoplasm with occasional short broad cell processes are frequently observed. Tumor cells with indistinct cell borders and subsequent syncytial whorls are the most commonly encountered cytologic characteristics. Siddiqui et al.2 suggested that the cytologic findings may not reflect the histologic subtype. However, Fukuoka et al.3 stressed that abundant and vacuolated cytoplasm and cystic spaces of various sizes, as well as rare nuclear inclusions or grooves, may be distinguishing findings in microcystic meningioma. In the current case, abundant vacuolated cytoplasm and subsequent low nuclear/cytoplasmic ratio were found; however, cystic spaces within the clusters and sheets were rarely found. Additionally, abundant bluish myxoid materials were notable in the background. The nature of the myxoid materials has been proven as a transudate of a low-protein component and several theories have been suggested, including recapitulation of subarachnoid structure, simple degenerative change, and disordered vascular permeability.5 To the best of our knowledge, these findings were not described in the case reported by Fukuoka et al.3 Cytoplasmic vacuolation and bluish myxoid materials in the background are features shared by chordoid meningioma.6 However, the latter is frequently associated with chronic inflammatory cells and cord-like epithelioid cells. The presence of background mucin-like bluish materials was an indication of possible mucin-producing metastatic adenocarcinoma, which could be excluded because most tumor cells were relatively monotonous and bland-looking in appearance with a smooth nuclear contour and no necrosis.2,7 In the current case, pleomorphic cells occasionally found on crush cytology were concordant with its histology. These pleomorphic tumor cells in microcystic meningioma may show xanthomatous changes, mimicking pleomorphic xanthoastrocytoma (PXA).8 Vacuolated cells, a characteristic cytologic clue for microcystic meningioma, are also found in PXA, hemangioblastoma, and angiomatous meningioma.9,10 Some pleomorphic cells in PXA may show rare intranuclear inclusions. However, the long, coarse fibrillary processes are helpful in distinguishing between refractile and hyaline cytoplasm of microcystic meningioma. In meningiomas showing extensive sclerosis like the current case, solid extracellular collagenous masses of variable size and well-defined edges were suggested as helpful cytologic diagnostic clues. Although no study indicating that microcystic meningioma can be accompanied by extensive sclerosis has been reported, its nature, such as rich vessels and rich myxoid edematous background, may explain sclerosing and fibrotic changes that occur during the course of the disease.
In summary, occasional cytologically pleomorphic mononuclear cells, hyalinized collagenous materials, bluish myxoid extracellular fluid, and rarely observed intracytoplasmic microcystic spaces posed a diagnostic problem in the current case. Despite limited experience, a careful search for extracellular myxoid fluid and vacuolated or microcystic cytoplasm may be helpful in its intraoperative diagnosis, even in cases of sclerotic microcystic meningiomas.
Notes
No potential conflict of interest relevant to this article was reported.