Expression of CD99 in Multiple Myeloma: A Clinicopathologic and Immunohistochemical Study of 170 Cases
Article information
Abstract
Background
Multiple myeloma (MM) is a heterogeneous and ultimately fatal disease. Risk stratification using prognostic biomarkers is crucial to individualize treatments. We sought to investigate the role of CD99, a transmembrane protein highly expressed in many hematopoietic cells including subpopulations of normal and neoplastic plasma cells, for MM risk stratification.
Methods
CD99 expression was measured in paraffin samples of bone marrow and extramedullary biopsies of 170 patients with MM. Patients were divided into those with high score (moderately and strongly positive) and low score (negative and weakly positive), with all staining being cytoplasmic and/or membranous.
Results
High anti-CD99 immunostaining was observed in 72 of 136 (52.9%) bone marrow biopsies and 24 of 87 (27.6%) extramedullary biopsies in MM. High CD99 expression of extramedullary specimens was associated with significantly longer overall survival (OS; p=.016). High CD99 expression of extramedullary specimens was also associated with better prognosis in the nonautologous stem cell transplantation group of MM patients (p=.044). In multivariate analysis, International Staging System stage was an independent prognostic factor, whereas CD99 expression was no longer statistically significant.
Conclusions
Expression of CD99 in extramedullary specimens was correlated with longer OS, suggesting that CD99 may be a helpful immunohistochemical marker for risk stratification.
Multiple myeloma comprises 10% to 15% of patients with hematologic malignancies.1 It is a slowly progressive, but fatal lesion (median survival, 3 to 4 years) without efficient curative regimens established to date. The standard treatment approach for multiple myeloma patients aged ≤65 years consists of high-dose chemotherapy followed by autologous stem cell transplantation (ASCT).2 The widespread use of ASCT and the introduction of the novel agents bortezomib and the immunomodulatory derivatives, thalidomide and lenalidomide, have significantly improved survival of patients with multiple myeloma.3,4 However, patients eventually become resistant to treatment and succumb to this disease.
One of the major obstacles in developing effective treatments for multiple myeloma is the heterogeneity of this disease. Although patients often present with the same histological characteristics, multiple myelomas have been classified into seven or eight subgroups by gene expression profiling or by cyclin D expression and chromosome translocation patterns.5,6 Each subgroup showed marked variation in clinical characteristics and treatment responses, requiring different therapeutic approaches.7 Although application of more aggressive treatment strategies in select high risk patient groups is crucial, few markers have been identified for subtyping based on risk stratification. Prognostic factors of multiple myeloma include International Staging System (ISS) score,8 extent of organ involvement,9 cytogenetic abnormalities,10,11,12 and some hematologic indices.13,14 However, there are only a few immunohistochemical markers in pathological specimens that are known to predict patient outcome.
CD99 is known to be a useful marker in the diagnosis of Ewing sarcoma and primitive neuroectodermal tumors.15 It is also expressed in many hematopoietic neoplasms, including lymphoblastic lymphoma/leukemia,16,17 acute myelogenous leukemia,18 anaplastic large cell lymphoma (ALCL),19,20 and diffuse large B-cell lymphoma (DLBCL).21 Furthermore, expression of CD99 has been reported to be significantly higher in DLBCL patients with high risk.21 Additionally, expression of CD99 was found in some plasma cell neoplasms,22 but the prognostic impact of CD99 in these tumors has not yet been fully investigated.
Based on this previous data, we analyzed expression of CD99 in a large number of plasma cell neoplasms and correlated expression with various clinicopathologic parameters and prognosis. We found that higher CD99 expression in extramedullary specimens was associated with longer overall survival (OS) in patients with multiple myeloma, particularly those who did not undergo ASCT.
MATERIALS AND METHODS
Patients and samples
Samples were collected from 170 patients diagnosed with multiple myeloma (plasma cell myeloma) and 15 patients diagnosed with plasmacytoma of extramedullary tissue at Asan Medical Center from 2001 to 2008. To minimize the effects of potential confounders, only specimens obtained at initial diagnosis were collected. The specimens included 136 bone marrow biopsy specimens and 102 extramedullary tissue biopsy specimens (87 cases of multiple myeloma and 15 cases of plasmacytoma) and both bone marrow and extramedullary biopsy specimens were available from 53 patients with multiple myeloma. Medical records were reviewed for clinical features, radiologic, laboratory, and pathologic findings, as well as treatment applied and clinical outcomes. Histologic morphology was considered to be of the plasmablastic type when >30% of tumor cells were plasmablastic (patient characteristics are summarized in Table 1).
Immunohistochemistry and pathologic evaluation
Paraffin-embedded tissue samples were immunohistochemically stained for CD99 using DAKO Envision-plus kits (Dako, Glostrup, Denmark) following manufacturer's protocols. Briefly, formalin-fixed, paraffin-embedded sections with 4-µm thickness were de-paraffinized and dehydrated through a graded alcohol series, and antigen was retrieved by incubation in citrate buffer for 1 hour at 95℃. The sections were incubated with hydrogen peroxide for 5 minutes and with Dako cytomation protein block for 5 minutes at room temperature. The tissue samples were subsequently incubated with anti-CD99 antibody (1:100, DN16, DiNonA, Suwon, Korea) for 1 hour at room temperature, washed, and incubated with secondary antibody for 1 hour. Antibody binding was visualized by incubation in diaminobenzidine solution for 1 minute, and the sections were counterstained with hematoxylin.
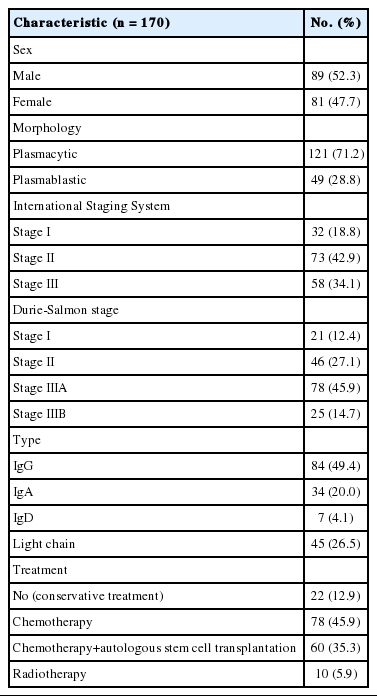
Immunohistochemical staining for CD99 was evaluated semi-quantitatively by assessing both the intensity and percentage of positive cells. The intensity of membranous or cytoplasmic staining was scored as negative (0), weakly positive (1), moderately positive (2), and strongly positive (3) (Fig. 1). Scoring based on percentage of positive cells was categorized as 0 (<10%), 1 (10% to 40%), 2 (41% to 70%), and 3 (71% to 100%). The final score was obtained by adding the scores for intensity and percentage. Each sample (bone marrow specimen or extramedullary specimen) was subsequently divided into two groups: those with low CD99 expression (score, 0 to 2; negative and weakly positive) and those with high CD99 expression (score, 3 to 6; moderately and strongly positive).
Statistical analysis
Correlations between expression of CD99 and categorical variables (age, sex, ISS stage, laboratory results, and bone lesion) were analyzed using Pearson's χ-square test or Fisher's exact test. Survival was analyzed by the Kaplan-Meier method. OS was defined as the time from the date of diagnosis by bone marrow or extramedullary tissue biopsy to the date of death, and recurrence-free survival was defined as the time from the date of diagnosis to the date of first recurrence. Patients lost to follow up or who died of other causes were left out of the analysis. Survival curves of two or more groups were compared by the log-rank test and Cox proportional hazards model. A p<.05 was considered statistically significant. All statistical analyses were performed using SPSS ver. 18 (SPSS Inc., Chicago, IL, USA).
Ethical permission
The Institutional Review Board (IRB) of Asan Medical Center (Seoul, Korea) approved the study protocol and provided all necessary ethical permissions.
RESULTS
Patient characteristics in plasma cell neoplasm
Of the 185 plasma cell neoplasms, 170 cases were multiple myelomas (plasma cell myeloma) and 15 cases were plasmacytoma, including nine extramedullary (extraosseous) plasmacytomas and six solitary plasmacytomas of the bone (solitary osseous plasmacytoma). The mean age of patients with multiple myeloma was 60 years (range, 29 to 84 years). The mean ages of patients with extramedullary (extraosseous) and solitary osseous plasmacytomas were 56 years (range, 28 to 74 years) and 57 years (range, 24 to 67 years), respectively. The male to female of patients with multiple myeloma was 1.09:1.
The clinical characteristics of the 170 multiple myeloma patients are summarized in Table 1. Laboratory results, including serum calcium, protein, and β2-microglobulin concentrations and 24-hour urinary protein, differed widely.
CD99 expression in plasma cell neoplasms
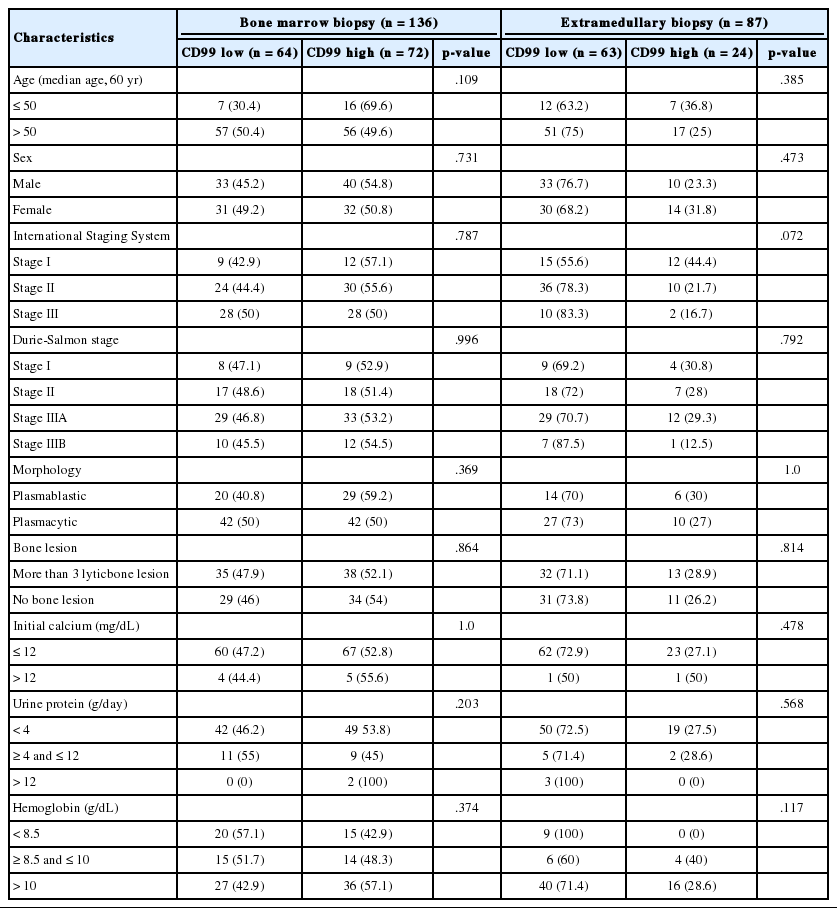
Immunohistochemical staining for CD99 showed a membranous and/or cytoplasmic staining pattern. Most CD99 positive bone marrow biopsies showed both cytoplasmic and membranous patterns, whereas most extramedullary biopsies showed a membranous pattern. In multiple myeloma samples, high anti-CD99 immunostaining was observed in 72 of 136 (52.9%) bone marrow and 24 of 87 (27.6%) extramedullary biopsies and there was no significant difference in CD99 expression (p=.365). Both bone marrow and extramedullary biopsy specimens were available from 53 patients with multiple myeloma, and there was no significant distinction or concordance of CD99 expression pattern between consecutive extramedullary and bone marrow samples. In plasmacytoma samples, high anti-CD99 immunostaining was observed in six out of 15 (40%).
CD99 expression and survival in multiple myeloma patients
The median follow-up period was 999 days (range, 2 to 4,686 days). At the time of analysis, 129 patients (75.9%) with multiple myeloma had died. When the patients were divided into those with low (score, 0 to 2; negative and weakly positive) and high (score, 3 to 9; moderately and strongly positive) CD99 expression, high CD99 expression in extramedullary biopsies of patients with multiple myeloma tended to be associated with low ISS grade (p=.072). No other clinical prognostic markers were associated with CD99 expression in either bone marrow or extramedullary biopsies (Table 2).
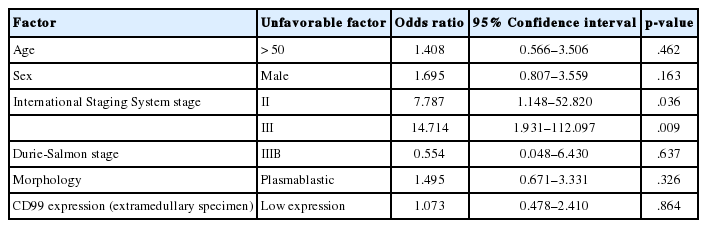
In the multiple myeloma patients with extramedullary biopsies (n=87), OS was significantly longer in the high CD99 expression (p=.016) group. However, OS was not associated with CD99 expression in multiple myeloma patients with bone marrow biopsies (n=136) (Fig. 2). Univariate analyses of multiple myeloma patients with extramedullary biopsy (n=87) showed that other factors related to poor OS included older age (p=.044), male sex (p=.022), plasmablastic morphology (p=.043), high ISS stage (p<.0001), and high Durie-Salmon stage (p<.0001). Multivariate analysis showed that ISS stage (serum β2-microglobulin) was an independent prognostic factor, whereas high CD99 expression in extramedullary biopsies was not statistically significant (p=.864) (Table 3).
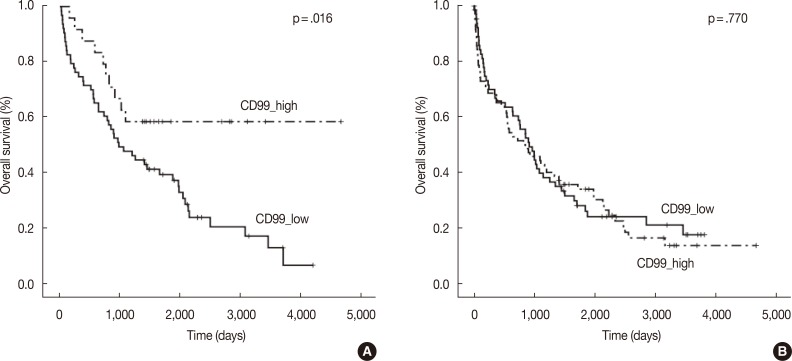
Kaplan-Meier survival curves in multiple myeloma patients with extramedullary biopsy (n=87) containing low CD99 expression (n=63) and high CD99 expression (n=24) (A), and bone marrow biopsy (n=136) containing low CD99 expression (n=64) and high CD99 expression (n=72) (B).
We also assessed CD99 expression related to ASCT response. Subdivision of patients with low CD99 expression in extramedullary biopsies demonstrated that OS was significantly longer in patients who had undergone ASCT (p=.004). Among patients who did not undergo ASCT, those with high CD99 expression had better OS than those with low CD99 expression (p=.044). Of patients who underwent ASCT, those with high CD99 expression also showed longer OS, but this difference was not statistically significant (p=.476). In patients with high CD99 expression, there was no survival difference between patients who did and did not undergo ASCT (p=.710) (Fig. 3A).
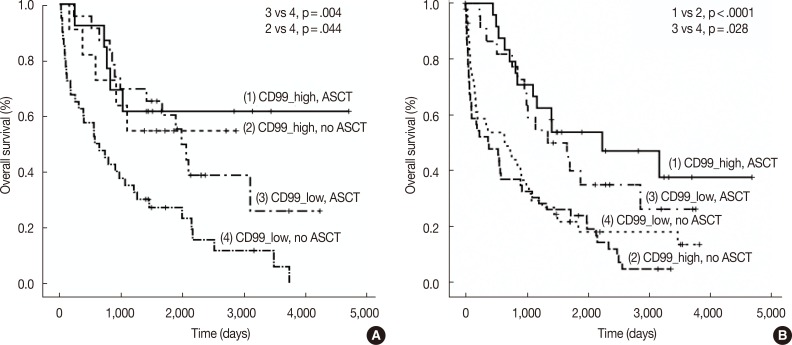
Kaplan-Meier survival curves in multiple myeloma patients with extramedullary biopsy (n=87) containing low CD99 expression with no autologous stem cell transplantation (ASCT) (n=40), low CD99 expression with ASCT (n=23), high CD99 expression with no ASCT (n=11), and high CD99 expression with ASCT (n=13) (A), and bone marrow biopsy (n=136) containing low CD99 expression with no ASCT (n=42), low CD99 expression with ASCT (n=22), high CD99 expression with no ASCT (n=48), and high CD99 expression with ASCT (n=24) (B). (A) 1 vs 2, p=.710; 3 vs 4, p=.004; 1 vs 3, p=.476; 2 vs 4, p=.044. (B) 1 vs 2, p<.0001; 3 vs 4, p=.028; 1 vs 3, p=.419; 2 vs 4, p=.332.
When we assessed OS in patients from whom we obtained bone marrow biopsies, we found that CD99 expression did not significantly affect patient survival, both in patients with (p=.419) and without (p=.332) ASCT. OS was significantly longer in patients who underwent ASCT than in those who did not, irrespective of CD99 expression (high CD99 group with ASCT vs high CD99 group with no ASCT, p<.0001; low CD99 group with ASCT vs low CD99 with no ASCT, p=.028) (Fig. 3B).
DISCUSSION
Recently, the expression and prognostic impact of CD99 were demonstrated in hematolymphoid neoplasms, including DLBCL, Burkitt lymphoma, CD30 negative peripheral T-cell lymphoma, and ALCL.19 We have investigated the prognostic significance of CD99 expression in multiple myeloma and assessed the utility of CD99 immunostaining as a histologic marker for risk stratification. In evaluating the correlation of CD99 expression with various clinical parameters and patient prognosis, we found that OS in patients with multiple myeloma was significantly associated with high CD99 expression in extramedullary specimens, particularly in patients without ASCT. Further, low CD99 expression in extramedullary specimens was associated with high ISS stage.
Our results partly correspond with previous studies. CD99 immunoreactivity has been associated with better prognosis or lower clinicopathological stages with urothelial carcinoma23 and neuroendocrine carcinomas of the pancreas, stomach, and lung.24,25 However, in lymphoid malignancy, CD99 positivity was reported to be associated with higher risks in patients with DLBCL21 and the non-germinal center B cell-like (GCB) subgroup of diffuse large B-cell lymphoma.26 This discrepancy may be due to several reasons. Multiple myelomas are indolent tumors that grow slowly, whereas DLBCLs are aggressive tumors that grow rapidly. The biological function of CD99 may therefore differ in these tumors, as the functions of CD99 are very diverse. CD99 expression was found to induce apoptosis,27 inhibit anchorage-independent growth, and result in anoikis resistance, and high expression of CD99 was found to contribute to the malignant properties of sarcomas by promoting growth.28 In hematopoietic cells, CD99 mediates T-cell interactions and induces apoptosis,29 whereas, in CD34+ progenitor cells, CD99 promotes cell proliferation and migration.30 Differences in the prognostic associations of CD99 may be due to differences in CD99 signaling pathways among cancer cell type. The function of CD99 and its signaling pathway are not completely understood, indicating the need for further studies of the function and biological significance of CD99 in multiple myeloma and plasma cells.
We also observed that the association between CD99 expression and patient survival of multiple myeloma differed in bone marrow and extramedullary specimens. There was no significant association between CD99 expression and patient survival in bone marrow samples of multiple myeloma. Discordant prognostic association between CD99 expression and different cancer subtypes has been reported previously. For example, CD99 expression was found to correlate with OS in patients with the GCB subtype of DLBCL, but showed an inverse correlation in patients with the non-GCB subtype.26 According to 2008 World Health Organization (WHO) classification of plasma cell neoplasms, extramedullary involvement of multiple myeloma is generally a manifestation of advanced disease, possibly representing different biological properties.13 CD99 may be involved differently in myeloma-stromal interactions in medullary and extramedullary sites.
Interestingly, we found that ASCT had no survival benefit in multiple myeloma patients with high CD99 expression in extramedullary specimens, whereas ASCT significantly improved OS in multiple myeloma patients with low CD99 expression in extramedullary specimens. By contrast, ASCT significantly enhanced OS in multiple myeloma patients with both high and low CD99 expression of bone marrow specimens. Aside from age, there are no criteria for selecting candidates for ASCT. Our finding that ASCT is associated with different survival outcomes in patients with high and low extramedullary expression of CD99 suggests that CD99 immunostaining in extramedullary specimens may be helpful in selecting patients for ASCT.
In conclusion, we have shown that extramedullary CD99 expression in multiple myeloma correlates with good prognosis. CD99 may be a useful immunohistochemical marker in risk stratification.
Acknowledgments
This study was supported by the Korea government (The national Research Foundation of Korea, MRC grant no. 2008-0062286) and the Asan Institute for Life Sciences (no. 2011-0794, Seoul, Korea).
Notes
No potential conflict of interest relevant to this article was reported.