Well-Differentiated Papillary Mesothelioma of the Tunica Vaginalis: A Case Study and Review of the Literature
Article information
Abstract
Well-differentiated papillary mesothelioma is an uncommon tumor of the testes that usually presents as a hydrocele. Here, we present the case of one patient who did not have a history of asbestos exposure. The tumor was localized in the tunica vaginalis and was composed of three pedunculated masses macroscopically. Microscopically, branching papillary structures with focal coagulative necrosis were present. In addition to immunohistochemistry, simian virus 40 DNA was also tested by polymerase chain reaction. This report presents one case of this rare entity, its clinical and macroscopic features, and follow-up results.
Mesothelial lesions of the paratesticular region include mesothelial cysts, reactive mesothelial hyperplasia, well-differentiated papillary mesothelioma (WDPM), and malignant mesothelioma (MM). Of these, WDPM is an uncommon tumor, usually detected in the peritoneum of women of reproductive age. It is infrequently seen in other anatomic sites, such as the pleura, pericardium, and tunica vaginalis. To our knowledge there have only been 19 well-documented case reports of paratesticular WDPM.1,2,3,4,5,6,7,8,9,10,11 Little is known about the clinicopathologic spectrum of this enigmatic neoplasm or of its overall prognosis. There is a need to identify more cases to better characterize the scope and spectrum of paratesticular WDPM. In this report, we present an additional case and discuss the clinical, radiologic, and histologic features in an attempt to better characterize this rare entity and to review the literature.
CASE REPORT
A 48-year-old man presented with a complaint of scrotal pain and swelling that had been gradually increasing for a period of 6 months. Physical examination revealed an enlarged left hemiscrotum. Testicular ultrasonography showed a left hydrocele consisted of three nodules measuring 2 cm, 1.4 cm, and 0.9 cm (Fig. 1) in the scrotal wall. Magnetic resonance imaging findings also revealed solid intrascrotal, extratesticular lesions. The patient had no history of trauma or asbestos exposure. A left radical orchiectomy was performed. The patient has done well with no signs of residual disease 3 years after surgery.
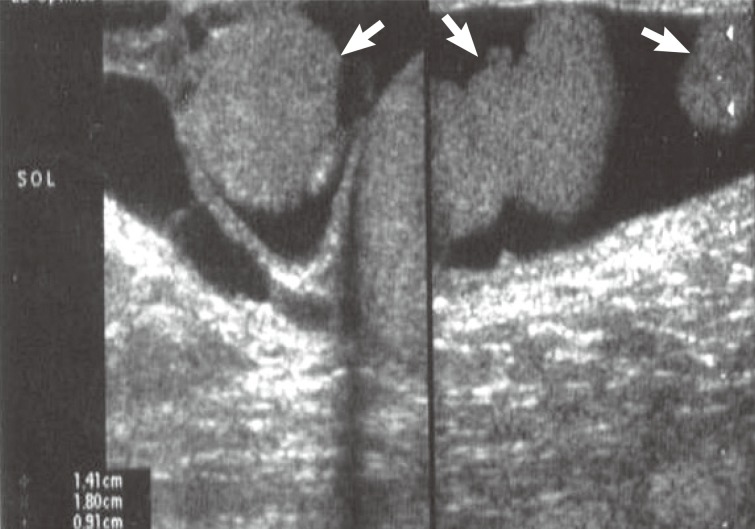
Ultrasonography shows three hypoechoic nodules measuring 2 cm, 1.4 cm, and 0.9 cm in the wall of hydrocele sac (arrows).
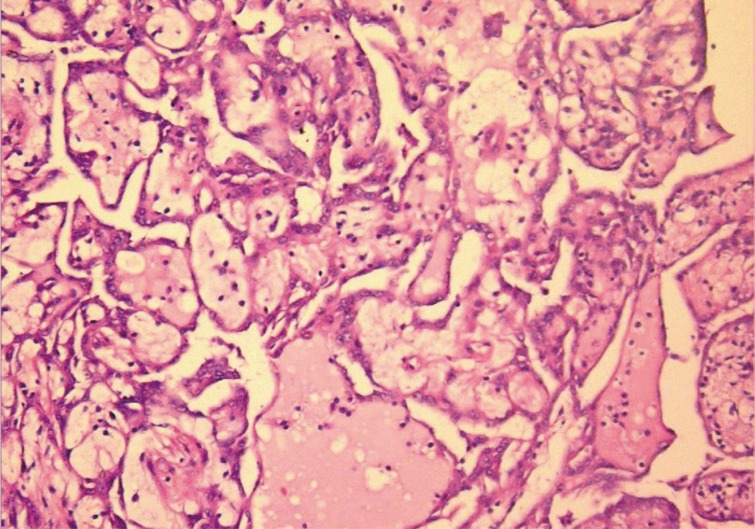
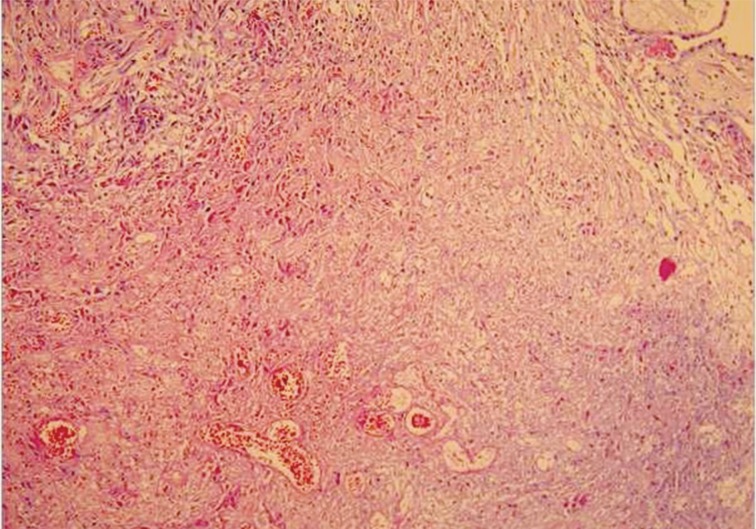
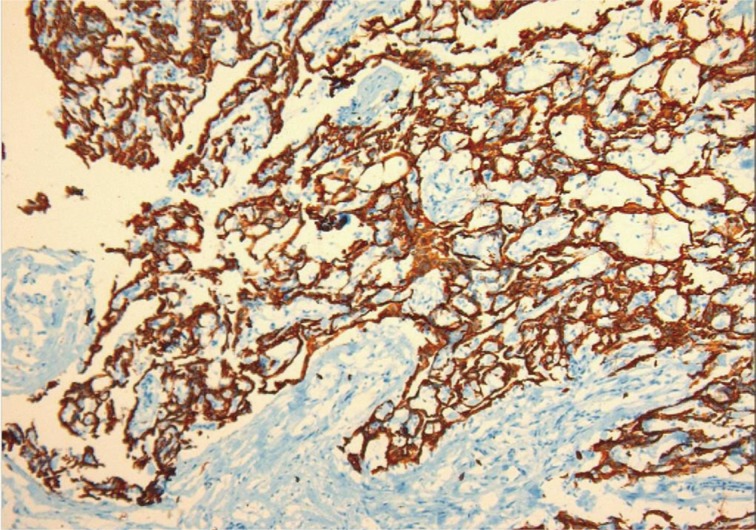
Macroscopically, the tunica vaginalis had three nodules. These were found to be pedunculated masses with 2 cm, 1.5 cm, and 1 cm in diameter. The testis was normal. Microscopically, the masses were composed of multiple branching, papillary structures with a fibrovascular core (Fig. 2). The papillary structures were lined with a single layer of bland cuboidal cells. The stroma was edematous and myxoid (Fig. 3). Focal areas showed a complex architectural pattern without subepithelial invasion. Focal coagulative necrosis was also seen (Fig. 4). There was no cytologic atypia or atypical mitosis. Immunohistochemical studies were performed. The lining cells were positive for calretinin (Fig. 5), epithelial membrane antigen, HBME-1, and cytokeratin 7. Negative results were found with carcinoembryonic antigen (CEA), cancer antigen 125, and CD10. Additionally, we searched for simian virus 40 (SV40) by polymerase chain reaction (PCR) amplification. DNA was tested using primers specific for the β-globulin gene. Two sets of primers (5'-GAA-TGG-GAG-CAG-TGG-TGG-AAT-GC-3' and 5'-TCT-CTT-CTT-TTT-TGG-AGG-AGT-AGA-3') were used for SV40 detection.12 No SV40 was detected in this case.

Tumor mass in the tunica vaginalis is composed of multiple branching, papillary structures with a fibrovascular core.
DISCUSSION
Mesotheliomas can arise from the pleura, peritoneum, or paratesticular areas, in decreasing order of frequency. Paratesticular mesothelioma can arise from the tunica vaginalis, formed by an outpouching of the abdominal peritoneum. Only 0.3-5% of cases of mesothelioma occur in the tunica vaginalis. Mesothelial lesions of this area include reactive mesothelial hyperplasia, mesothelial cysts, adenomatoid tumor, WDPM, and MM. WDPM is a rare type of mesothelioma that mostly arises from the peritoneum or pleura.13,14 Barbera and Rubino9 reported the first case of WDPM in 1957. To the best of our knowledge only 18 cases of paratesticular WDPM have been published to date in the English literature. These cases are summarized in Table 1. The majority of these cases have been presented as case reports, except for one series of 8 cases that was published by Brimo et al.8 recently. All reported cases had similar morphology with papillary/tubulopapillary architecture. All cases also had bland appearing cells which lack significant atypia, pleomorphism, and stromal invasion. Only Trpkov et al.6 mentioned a small focus of coagulative necrosis. A minority of cases showed psammoma bodies. One of the cases in the series by Brimo et al.8 showed osteoclast-like giant cells.
The main differential diagnosis includes mesothelial hyperplasia and MM. Mesothelial hyperplasia can also be composed of papillary structures, but often do not contain a fibrovascular core like WDPM. The papillary structures are often accompanied by inflammation and reactive changes. One major clinical concern is differentiating WDPM from MM, as they can have overlapping histologic features. The presence of invasion, marked cytologic atypia, atypical mitosis, and a bulky mass are the major features that should be considered in making a diagnosis.15,16
The etiology of WDPM has not been clearly elucidated. It has been suggested that proliferative lesions might be related to local trauma, herniorrhaphy, or a long-term hydrocele.17 A relationship between asbestos exposure and mesotheliomas of the tunica has been postulated. Only one case of WDPM of the tunica vaginalis in the literature had a history of asbestos exposure7 and one had a history of a household contact with exposure.4 Our case had no asbestos exposure like the rest majority of reported cases. In addition to asbestos, potassium bromate in drinking water has been shown to increase the risk of MM of the tunica vaginalis in an animal model.15 SV40 is a DNA monkey virus that has been associated with MM and has been implicated in mesotheliomas of the pleura. SV40 contaminated polio vaccines produced between 1955 and 1978 are postulated to have been the route of virus transmission from monkeys to humans. Many studies have demonstrated the presence of SV40 in pleural mesotheliomas through the use of various techniques, and animal studies have demonstrated a clear association of up to 60% of developing mesothelioma.15,16,18 However SV40 DNA has not been as well studied in mesotheliomas of the tunica vaginalis as it has been in its pleural counterpart. Xiao et al.1 applied PCR for SV40 DNA in their WDPM case and found it to be negative. We also performed PCR, but did not find SV40 in our case. In taking into consideration all reported cases, hydrocele seems to be the major risk factor for developing WDPM of the tunica vaginalis.
Non-mesothelial tumors of diverse histology must also be kept in mind in the differential diagnosis. This group includes papillary cystadenoma of the epididymis. Features that distinguish papillary mesothelioma from papillary cystadenoma include the absence of clear cells and the presence of calretinin positivity. Papillary serous neoplasms may also be difficult to differentiate from WDPM. However, serous tumors tend to have broader papillae and stratification, budding, and occasional cilia of tumor cells. Psammoma bodies are more numerous than WDPM. Additionally, papillary serous tumors express CEA and Ber-Ep4, both of which are usually negative in WDPM.19,20
The current case had a focal area of consisting of only superficial, entrapped tubules in the underlying tissue. Given that the stroma exhibited no evidence of hypercellularity, storiform growth or desmoplastic response, we did not consider the tumor to be invasive. Another challenging issue was focal necrosis. Only one previous report mentioned a small focus of coagulative necrosis.6 This area was considered coagulative necrosis in the current case due to torsion or infarct, as mentioned in the previous report. Currently, the main limitation in differentiating WDPM from MM with dominant papillary growth is limited experience, with only 18 known cases in the literature. Although our case had no invasion, cytologic atypia, infiltrative growth, or mitotic activity we recommended close patient follow-up because of the areas of focal necrosis and complexity. This patient has had 4 years of uneventful follow-up. The biologic behavior of paratesticular WDPM is not well-known because of such a limited number of cases. A large series is needed to answer the questions about this enigmatic tumor.
Notes
No potential conflict of interest relevant to this article was reported.