Inflammatory Myofibroblastic Tumor of the Thyroid Gland: A Brief Case Report
Article information
Inflammatory myofibroblastic tumor (IMT), also known as inflammatory pseudotumor (IPT) or plasma cell granuloma (PCG), is a histologically distinctive lesion that occurs primarily in the viscera and soft tissue, but has been reported in nearly every site in the body.1 Until now, only 19 cases of IMT that have involved the thyroid have been reported in the English literature.2 Moreover, most cases in the thyroid have been described as cases of PCG,2 and the myofibroblastic component in the thyroid cases is usually not prominent. Only one case has reported IMT of the thyroid gland.3 This study reports and discusses the differential diagnosis of an IMT of the thyroid gland that occurred in a 50-year-old Korean woman and which showed a predominantly myofibroblastic proliferation.
CASE REPORT
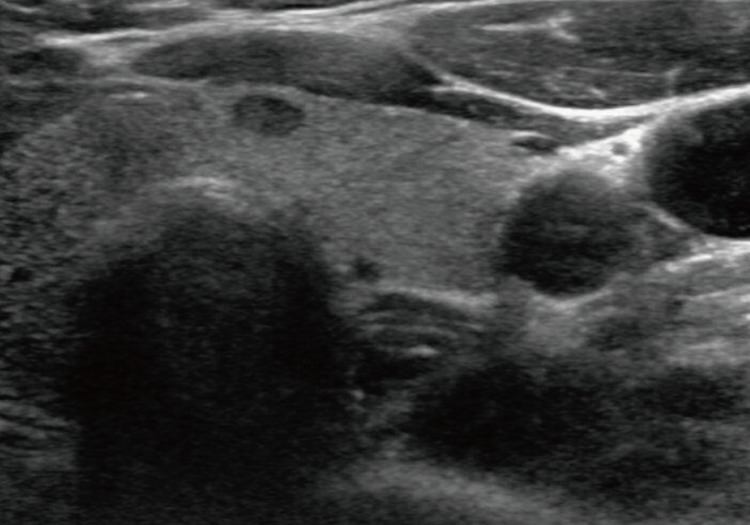
A-50-year-old female presented with a right thyroid mass and did not have a history of Hashimoto's thyroiditis. All routine laboratory findings and thyroid function tests were normal. Ultrasonography revealed a 0.6×0.6-cm lobulated hypoechoic mass in the right lobe and a 0.6×0.5-cm oval hypoechoic mass in the left lobe (Fig. 1). Fine-needle aspiration cytology of the right thyroid mass showed as a Hurthle cell type follicular neoplasm. Fine-needle aspiration or biopsy was not performed in the left thyroid. The patient underwent total thyroidectomy under general anesthesia.
The total thyroidectomy revealed two nodules in both lobes. A well-defined, solid, tan-colored encapsulated lesion that measured 0.6 cm at its greatest diameter was observed grossly in the right lobe. Histopathology revealed follicular adenoma. In the left lobe, a well delineated, but unencapsulated lesion that measured 0.6 cm at its greatest diameter was present (Fig. 2A). The lesion was characterized by poorly defined spindle cells with slightly pleomorphic nuclei (Fig. 2B). Histiocytes were frequent and often contained fine hemosiderin granules (Fig. 2C). There were a few lymphocytes or plasma cells and the lesion did not have any mitotic figures or lymphovascular invasion. The trapped residual thyroid follicular cells had no characteristic cytological findings of papillary carcinoma (Fig. 2D). The remaining thyroid tissue showed no pathological findings.
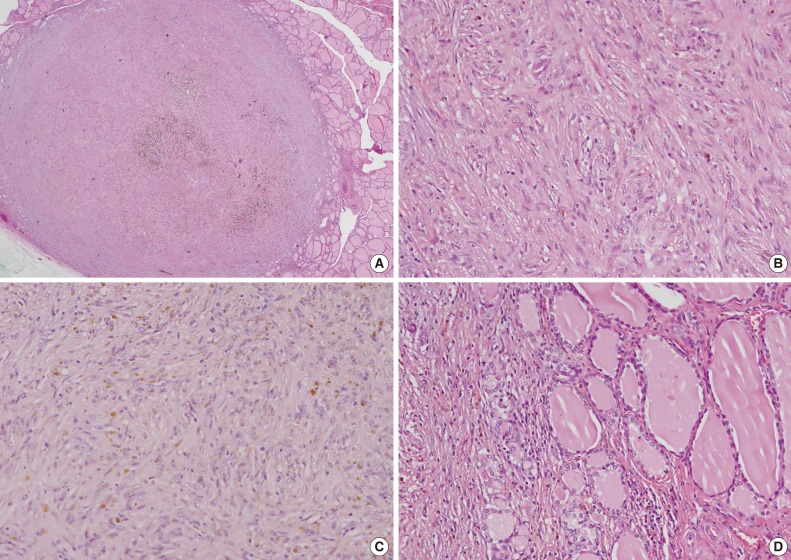
Histologic examination. (A) The greater part of the area is a well-circumscribed lesion without capsulation. (B) Spindle cells show ovoid and slightly pleomorphic nuclei, and the nucleolus are small but distinct. There are also lymphocytes and a few plasma cells and mitosis is not clear. (C) Hemosiderin-loaded histiocytes and small lymphocytes are mixed with haphazardly scattered spindle cells. (D) The trapped residual thyroid follicular cells have no characteristic cytological findings of papillary carcinoma.
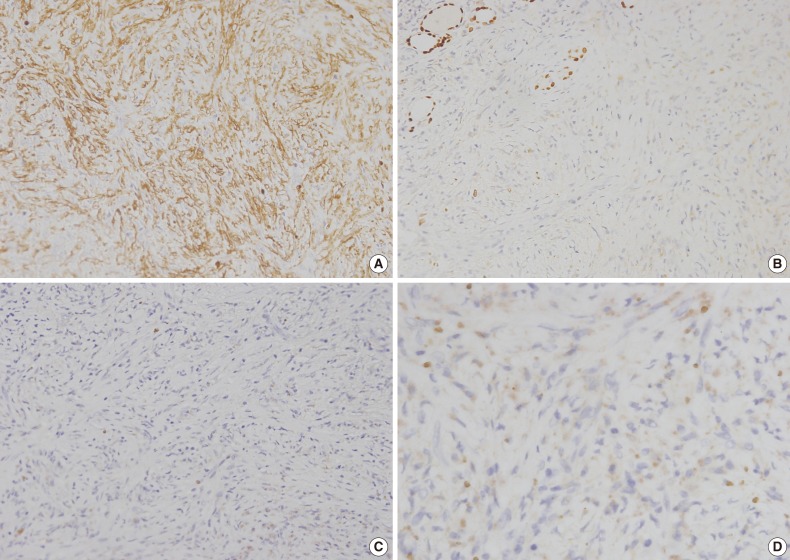
Immunohistochemical analysis indicated that the spindle cells were positive for α-smooth muscle actin, but negative for cytokeratin (CK), CK19, epithelial membrane antigen, thyroid transcription factor-1, CD34, desmin, anaplastic lymphoma kinase (ALK), and Bcl-2 (Fig. 3A-C). Hemosiderin-loaded histiocytes and a few spindle cells were positive for CD68. The MIB-1 labeling index was below 1% in the proliferated spindle cells. Plasma cells were rarely observed and negative for immunoglobulin G4 (IgG4) (Fig. 3D). Immunohistochemistry revealed that the spindle cells had myofibroblastic features. A diagnosis of IMT was proposed based on the immunostains and morphology. The patient had an uneventful postoperative course and was doing well one year after surgery.
DISCUSSION
IMT is composed of myofibroblastic spindle cells and an inflammatory cell infiltrate of plasma cells, lymphocytes and eosinophils.1 IMT occurs primarily in the viscera and soft tissue, but can occur at any anatomic location.1 Various terms have been used to reference IMT, including PCG, IPT, and IMT and have been used synonymously in most publications. Therefore, thus we reviewed all 19 cases that have been diagnosed in the English literature as PCG, IPT, or IMT of the thyroid gland (Table 1).2 In comparison with IMT in other sites, most reported thyroid cases showed a prominent plasma cell infiltrate embedded in a variable degree of fibrous stroma. Only two of the reported cases exhibited morphologic features of IMT and one of those cases was reported as IMT of the thyroid gland.3,4 Extrapulmonary IMT affects children and young adults within the first two decades of life, and is rare after 30 years.1 However, the clinicopathological findings of IMT of the thyroid gland appear to be different to other IMT findings. Thyroid gland IMT predominantly occurred in females (14/19), with an average age of 51 years (range, 18 to 89 years).2 This report describes an IMT of the thyroid gland that showed predominantly myofibroblastic proliferation that occurred in a 50-year-old woman. This is the first case of a thyroid gland IMT in Korea.
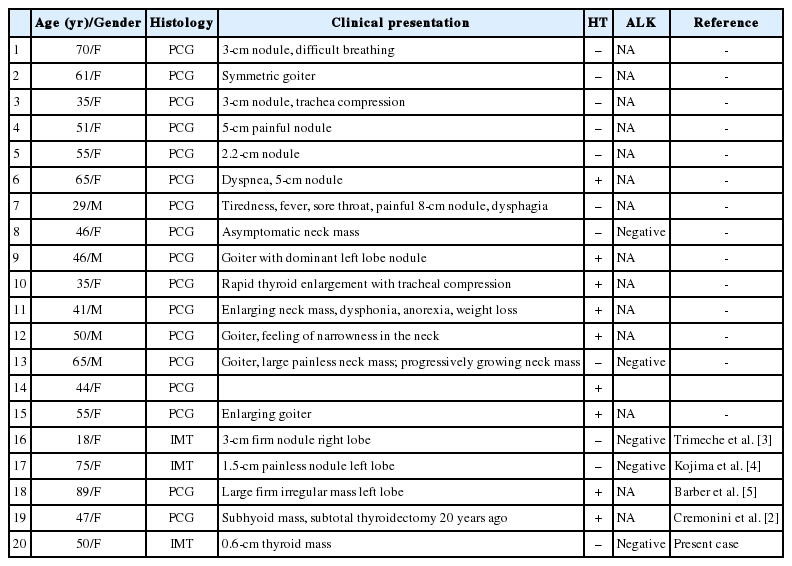
Main clinicopathologic findings of thyroid inflammatory myofibroblastic tumors/plasma cell granulomas in the English literature
The pathogenesis of IMT remains unclear although various allergic, immunologic, and infectious mechanisms have been postulated.2 Nine of the 19 reported cases of IMT of the thyroid gland were associated with Hashimoto's thyroiditis.2 These 9 cases were reported as PCG of the thyroid. This finding supports the hypothesis that PCG may employ a pathogenic mechanism that involves autoimmune processes.2 The mechanism, however, remains unclear in cases of IMT. Kojima et al.4 reported one case of IPT with predominant fibrohistiocytic proliferation and no history or pathological findings of Hashimoto's thyroiditis.
About 36% to 60% of extrapulmonary IMTs show ALK gene rearrangements, supporting their neoplastic origin.1 Of the 19 cases of thyroid IMT, ALK immunohistochemistry was performed in five cases, and all of the cases were determined to be negative.2 In this case, the tumor cells also showed negative immunoreactivity with ALK protein. The presence of ALK protein expression essentially serves as a surrogate marker for translocation that involves the ALK locus at chromosome 2p23, hence excluding a reactive myofibroblastic proliferation. These findings suggest that thyroidal IMT is a reactive rather than neoplastic lesion.
The case in this study was predominantly characterized by myofibroblastic proliferation, and this specific type of lesion should be differentiated from various non-neoplastic or neoplastic lesions that show spindle cell proliferation.1,2 Extensive fibrosis may be encountered in some forms of thyroiditis, such as the fibrous variant of Hashimoto's thyroiditis and Riedel's thyroiditis, although sampling can distinguish between such lesions and IMT. This lesion did not exhibit any of the typical signs of thyroiditis, and solitary fibrous tumors rarely affect the thyroid gland. Solitary fibrous tumor was excluded as a diagnosis due to the lack of hemangiopericytoma-like areas and strong CD34 immunoreactivity.1 Papillary thyroid carcinoma can be associated on rare occasions with an exuberant nodular fasciitis-like stroma,6 giving rise to a microscopic picture that may resemble a benign spindle cell proliferation. However, the trapped residual non-neoplastic thyroid follicular cells did not show any characteristic cytological findings of papillary carcinoma in this case report. Thyroid IMT reported as PCG also should be distinguished from IgG4-related sclerosing disease,2 and the possibility of IgG4-related sclerosing disease was ruled out in this case by histological and immunohistochemical evaluation. The analysis revealed the presence of a few plasma cells, which were negative for IgG4 immunohistochemical staining.
Characterization of IMT has evolved over time; initially it was considered as a benign reactive inflammatory process to a neoplasm of intermediate biological potential, with frequent recurrences and occasional metastasis.1 However, the clinical behavior of thyroid gland IMT differs from that of extrathyroidal IMT. All reported cases of thyroid gland IMT were benign and there were no incidences of recurrance.2 Surgical excisions is currently the method of choice for IMT treatment. Satisfactory results have also been obtained with steroids or L-thyroxine.5 Our patient underwent surgery and was doing well with no evidence of disease 1 year after the surgery.
In conclusion, thyroidal IMT is a rare tumor composed of a proliferating myofibroblastic spindle cells. IMT can usually be treated successfully with surgery. Therefore, it is important to accurately diagnose IMT to avoid unnecessary aggressive treatment.
Notes
No potential conflict of interest relevant to this article was reported.