Solid Form of Epithelioid Hemangioma: A Case Report
Article information
Epithelioid hemangioma (EH) is an uncommon benign vascular tumor, of controversial etiology that usually presents as a slowly growing nodule on the face or digit of a middle-aged woman[1]. Most lesions have a nonspecific nodular appearance with frequent secondary changes such as excoriation and bleeding. Multiplicity is also a common finding. Histopathologically, EH is characterized by a mixture of vascular proliferation and marked mixed inflammatory cell infiltration, and was first described by Wells and Whimster[2] as angiolymphoid hyperplasia with eosinophilia. The proliferation of vascular structures lined with prominent endothelial cells is a distinguishing feature of EH. The epithelioid endothelial cells that protrude into the vascular lumen create a characteristic “cobble-stone” or “tombstone” appearance. A chronic inflammatory cell infiltration including lymphoplasma cells and eosinophils is a consistent finding. EH has a diverse range of microscopic features, depending on the composition and distribution of the vascular and inflammatory cellular components. EH has been described in the literature as an inflammatory angiomatous nodule or, an atypical or pseudopyogenic granuloma[3,4], when infiltration of various inflammatory cells is predominant, and a histiocytoid hemangioma5 when cobble stone-like endothelial cells are conspicuous.
Some cases of EH consist entirely of solid sheets of epithelioid to spindled cells without fully canalized vascular structures. The solid form of EH can be difficult to diagnose and is occasionally misdiagnosed as a malignant vascular tumor. EH is a benign neoplasm and surgical excision is sufficient for its treatment. Recently, we experienced a solid form of EH with no inflammatory component, which showed a dramatic change into the typical morphology on the consecutive biopsy. This is the first reported Korean case of a solid form of EH, which pathologists should include in the differential diagnosis of epithelioid vascular lesions to avoid overdiagnosis of epithelioid vascular malignancies.
CASE REPORT
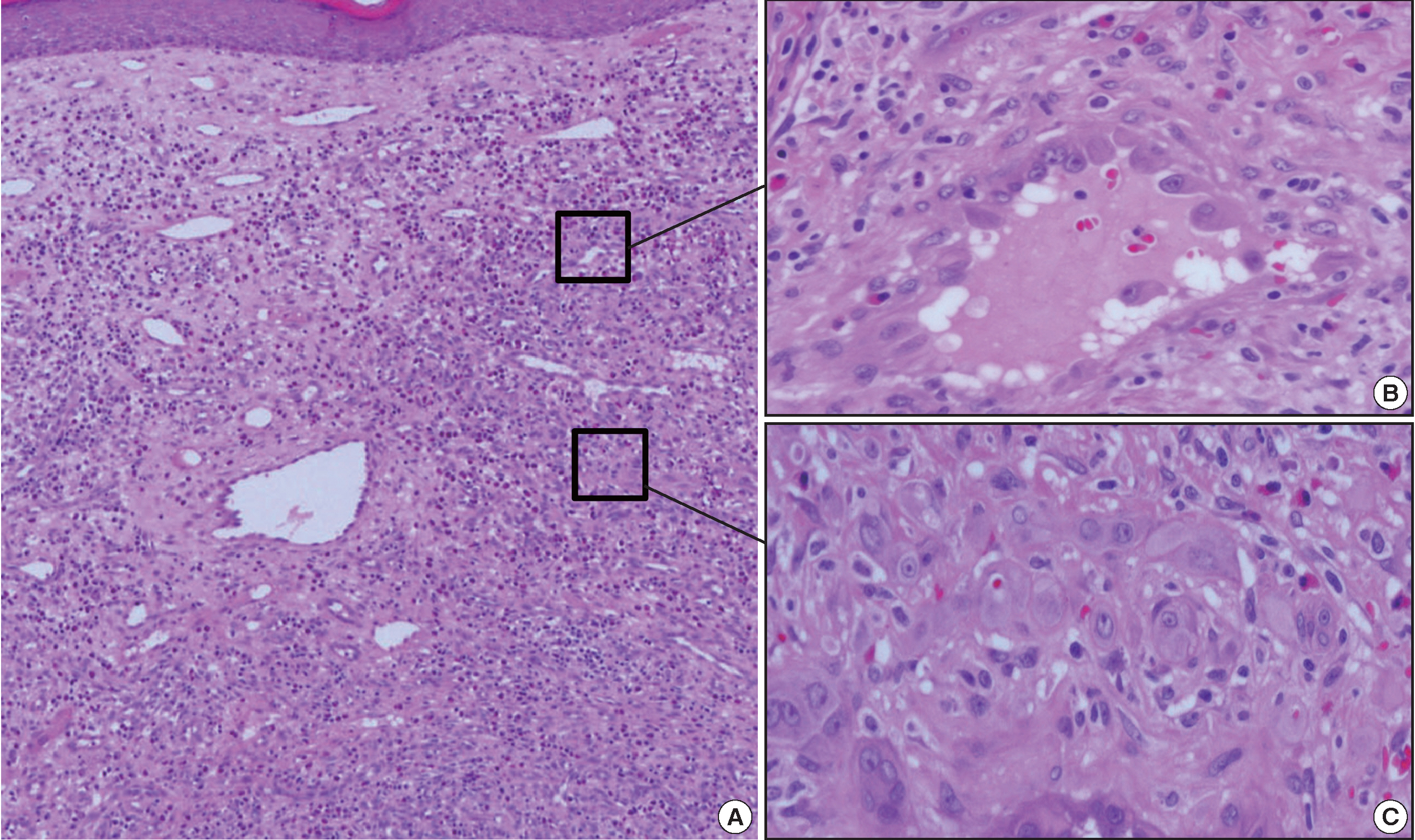
A 35-year-old female patient presented with multiple consecutive dermal nodules in her left forearm. On the initial physical examination, there were approximately ten well-defined, erythematous, round nodules up to 0.6 cm in size. The lesions were nontender, nonpruritic, and without ulceration (Fig. 1). The lesions slowly grew in size over the course of 4 months. The patient was otherwise healthy with no significant medical conditions. Excisional biopsies were performed in three nodules in the left forearm and elbow area. Microscopic examination of skin biopsies of the lesions revealed ill-defined lobular architecture with focally infiltrative borders in the superficial dermis (Fig. 2A). Most of the lesions consisted of solid sheets of epithelioid and spindle cells. Those cells had a moderate amount of eosinophilic cytoplasm, and some had cytoplasmic vacuoles (Fig. 2B). They had moderately pleomorphic nuclei and prominent nucleoli. Some of the epithelioid cells formed immature vessels. Vascular structures of variable size were identified at the periphery of the lesion (Fig. 2D). The vascular spaces were canalized and lined by plump epithelioid endothelial cells. Few inflammatory cell infiltrations were visualized and extravasated red blood cells were prominent. No necrosis was present. Up to five mitotic figures per high-powered field were identified with no atypical mitoses. These cells were focally positive for CD31 and CD34, but negative for human herpesvirus-8 (HHV-8) on immunohistochemical staining (Fig. 2C, E). Resection margins were clear. The initial presumptive diagnosis was epithelioid vascular tumor of borderline malignancy.
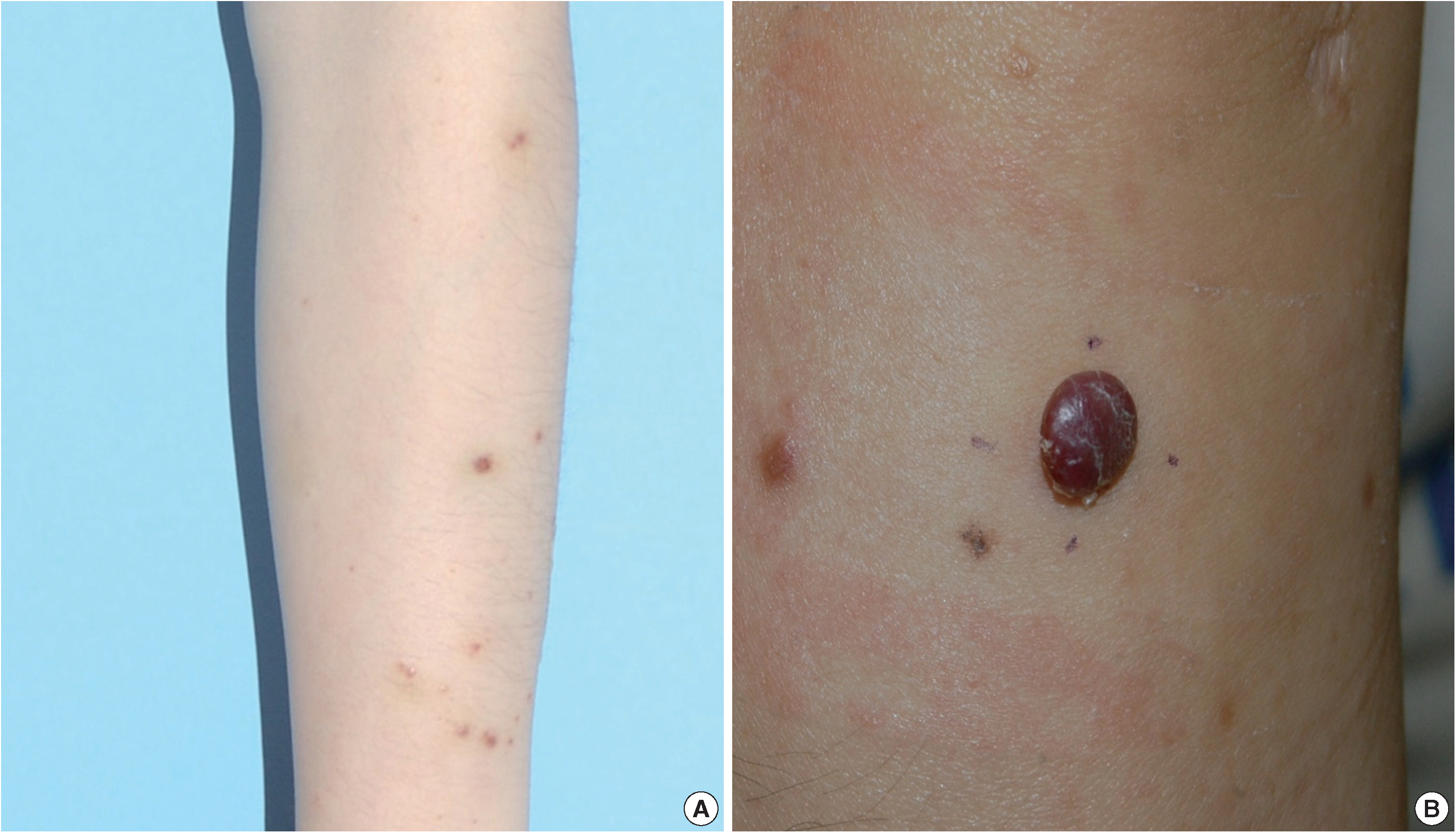
(A) Multiple well-defined erythematous papules on the forearm, each measuring 0.3–0.4 cm. (B) A recurrent erythematous, round protruding papule in the forearm, measuring 0.7 cm.

Histologic features of the first lesion. (A) The lesion is a poorly differentiated cellular tumor with focally infiltrating borders in the superficial dermis. (B) Most of the lesion shows solid proliferation of epithelioid endothelial cells. (C) Epithelioid endothelial cells are focally positive for CD34 immunohistochemical staining. (D) At the periphery, well-canalized vessels are observed. (E) Human herpesvirus-8 related antigen is negative.
Approximately 1 year later, a 0.7-cm-sized nodule with the same clinical features as the nodules seen on initial physical exam developed. Histologically, this was a superficial ill-defined lesion with remarkably different morphologies to previous lesions (Fig. 3A). Most of the lesion demonstrated typical histologic features of EH (Fig. 3B). Vascular structures had prominent endothelial cells protruding deeply into the lumen, creating “tombstones” appearance. Mixed inflammatory cells, including many eosinophils, were dispersed between the tumor cells. A central, focal, and solid component similar to the previous biopsy was present (Fig. 3C). The epithelioid endothelial cells were also immunopositive for CD31. The cells expressed Factor VIII and CD34 to a much lesser extent than CD31. D2-40 immunostaining was negative (data not shown). The patient has survived more than 30 months since the first biopsy. There has not been any recurrence of the lesions after application of a topical corticosteroid.
Histologic features of the second lesion. (A) A recurrent lesion shows typical histologic characteristics of epithelioid hemangioma. (B) Vascular structures that comprise the lesion are lined by prominent epithelioid endothelial cells. The stroma contains mixed inflammatory cells, including many eosinophils. (C) A focal solid component is present in the center of the lesion.
DISCUSSION
In this paper, we present the case of a patient with an EH comprised almost entirely from epithelioid endothelial cells with a solid growth pattern. The differential diagnoses for cutaneous lesions that mostly consist of epithelioid cells include poorly differentiated squamous cell carcinoma, melanoma, epithelioid vascular tumor[6], atypical fibroxanthoma, cutaneous leiomyosarcoma, epithelioid fibrous histiocytoma, and epithelioid sarcoma. Immunohistochemical stains are crucial in classifying the lineage of the tumor. However, immunohistochemical stains do have limitations. For instance, certain melanomas, atypical fibroxanthomas, and even carcinomas can express CD31[7] and some vascular tumors such as epithelioid angiosarcoma (EA) express vascular markers only focally or weakly, but may express cytokeratin[7].
Among the epithelioid vascular tumors, major differential diagnoses are anaplastic Kaposi sarcoma (KS), epithelioid hemangioendothelioma (EHE), and EA. Anaplastic KS is rare variant of KS. Histologically it shows a typical haphazard proliferation of epithelioid and spindle cells with moderate pleomorphism. Unlike EH, it displays marked cellular atypia and brisk mitosis. A few reports have indicated its aggressive clinical behavior[8]. Immunohistochemical staining for HHV-8 confirm the diagnosis. The tumor in this case was negative in HHV-8 immunostaining and exhibited benign clinical behavior. EHE usually arises as a solitary mass on the extremities. The tumor cells arrange in cords or chains in distinctive myxohyaline stroma. Most cases of EHE follow an indolent clinical course, with 20% to 30% of cases showing risk of metastasis[9]. In this patient, diagnosis was potentially confusing owing to the proliferation of solid endothelial cells. However, the mass did not have the typical myxohyaline or sclerotic stroma of EHE. EA can also display solid sheets of epithelioid or spindled endothelial cells without obvious vasoformation. Although EA is most prevalent during the seventh decade of life, it can affect individuals of all ages. Patients often present with a rapidly enlarging mass deep within the muscles of the lower extremities. Important histologic characteristics to discriminate EA from other vascular tumors are severe nuclear atypia, brisk mitotic counts, coagulative necrosis, and extensive hemorrhage. EA is a highly malignant tumor and more than half of patients die within the first year. In our case, histologic features such as a lobular architecture, peripheral maturation, absence of necrosis or atypical mitotic figures, and the lack of significant nuclear atypia decreased the index of suspicion for high-grade malignancy. Its clinical behavior was also indolent.
In summary, a lesion composed of epithelioid endothelial cells in a solid growth pattern can be considered EH when there are none of the above mentioned evidences of malignancy, characteristic myxohyaline stroma, or immunoreactivity for HHV-8.
An EH showing an entirely solid growth pattern, as in this case, is extremely rare. A review of the published literature written in English identified, only one study on the solid form of EH, which described these lesions as an atypical, immature, or exuberant form of EH[10]. However, EH with focal solid growth is not uncommon if the mass is scrutinized carefully. One study reported that 80% of solid form of EH cases were interpreted as malignant vascular tumors by at least one contributing pathologist[10]. Therefore, using a punch biopsy that may include only part of the solid mass can cause a diagnostic challenge for pathologists who are not aware of solid form of EH. To our knowledge, our present study is the first report of a solid form of EH in the Korean pathologic literature. It is important that the solid form of EH be distinguished from malignant vascular tumors to avoid overly aggressive intervention.
Notes
No potential conflict of interest relevant to this article was reported.