Myoepithelial Carcinoma of Soft Tissue: A Case Report and Review of the Literature
Article information
Myoepithelial carcinoma of soft tissue is extremely rare although its counterpart of the salivary gland is relatively common and well-known with similar morphology. The histogenesis of myoepithelial carcinoma of soft tissue is still unknown. In fact, the tumor may present with myoepithelial differentiation but not originate from myoepithelial cells [1]. The tumor cells are heterogeneous in terms of cell type and architecture. The tumor cells may be epithelioid, spindled, clear, or plasmacytoid. The growth pattern varies and can be solid sheets, reticular, or trabecular architecture without ductal differentiation [2]. Myoepithelial carcinoma usually shows cytologic atypia, mitotic activity, infiltrative growth, or tumor necrosis [2]. Only few studies have been reported because of the rarity of myoepithelial carcinoma of soft tissue [1-4]. Consequently the clinical and pathologic characterization has been limited. Also, the limitation of definite diagnostic criteria and prognostic parameters makes the diagnosis and management of myoepithelial carcinoma of soft tissue difficult. Here we report the first case of myoepithelial carcinoma of soft tissue in Korea.
CASE REPORT
A 69-year-old male presented with a palpable mass in the right shoulder area. The mass had been noted 6 months prior and the size of the mass had recently increased. On physical examination, the round and firm mass was fixed on the upper aspect of the right scapula without tenderness. The sonography revealed a heterogeneous echoic solid mass. A local excision was performed. The mass was located in the subcutaneous soft tissue just above the trapezius muscle and measured 4.0×3.5×2.0 cm.
The solid mass was lobulated and the cut surface of the mass was gray tan with multiple necrotic foci (Fig. 1A). The tumor was confined to the subcutaneous soft tissue without dermal invasion. Although the tumor showed a well-circumscribed lobulating border, microscopic tumor infiltration into the adjacent soft tissue was observed (Fig. 1B). The tumor presented with various histologic growth patterns including solid sheet, trabecular, reticular patterns, and short fascicle with myxoid and hyalinized stroma. Although tumor cells had a heterogeneous cytomorphology consisting of epithelioid cells, spindle cells, clear cells, and plasmacytoid cells, the tumor cells generally showed vesicular or coarse chromatin, distinct nucleoli, and thick irregular nuclear membrane (Fig. 2). Numerous mitoses were noted (up to 42 per 10 high-power fields). In addition, multifocal tumor necrosis was found. Lymphovascular invasion was also found.
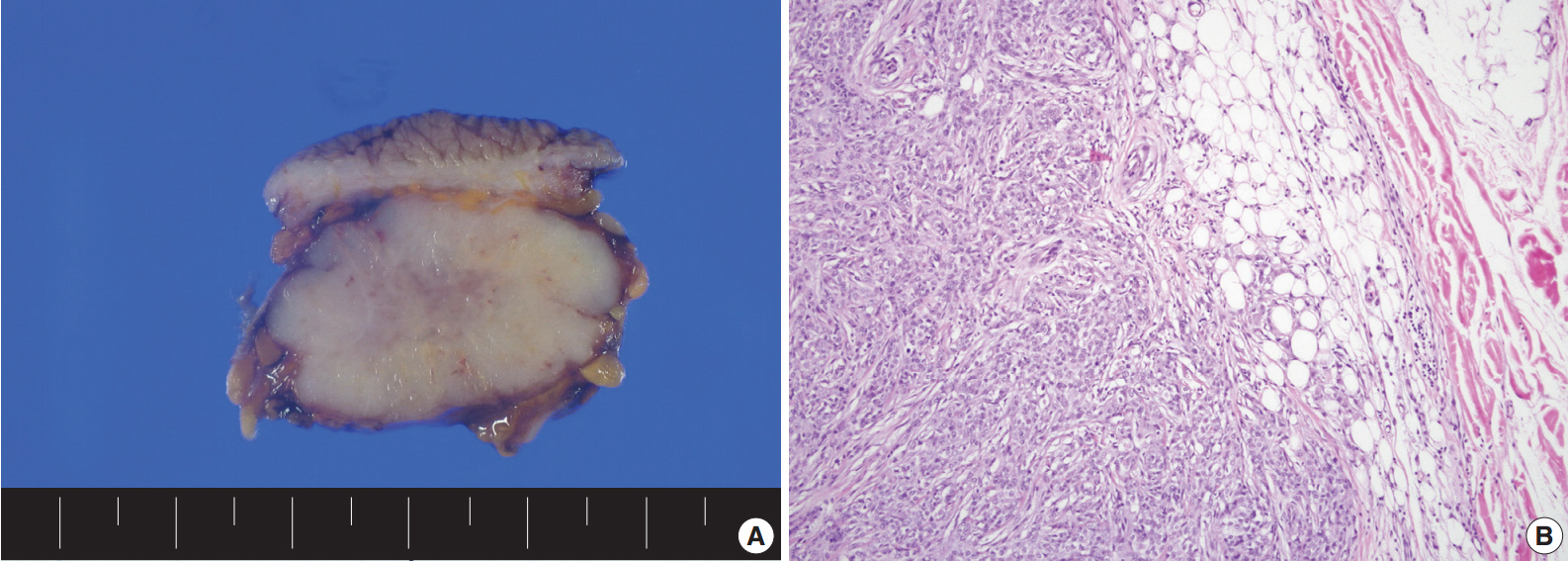
(A) A well-circumscribed lobulating tumor is noted in subcutaneous soft tissue with gray tan cut surface and multiple necrotic foci. (B) Microscopic infiltration into the adjacent soft tissue is found.
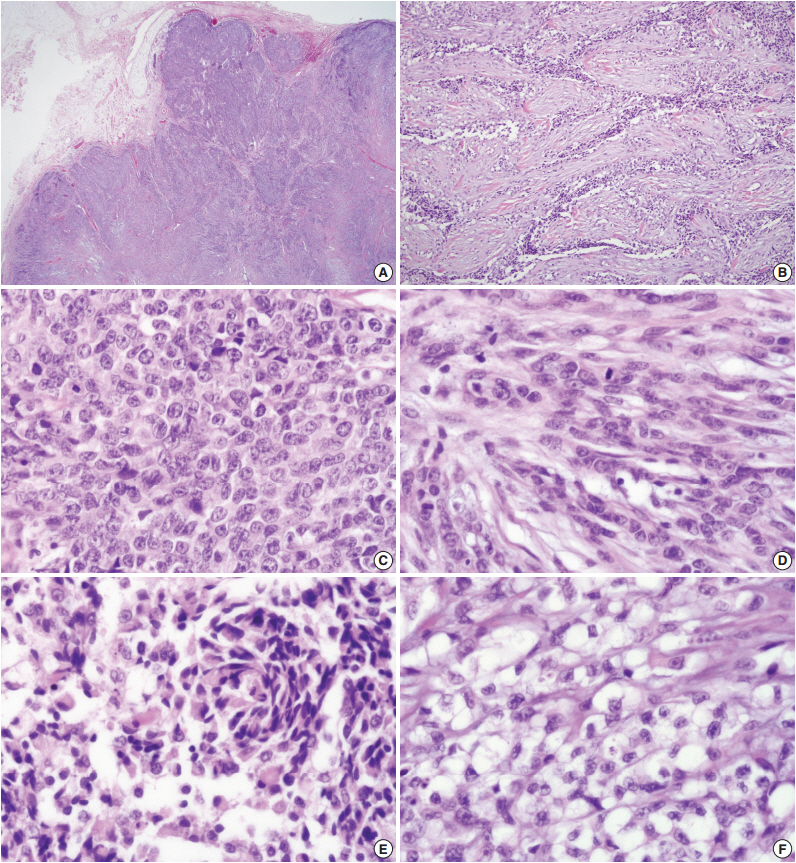
The tumor shows lobular architecture (A) and reticular pattern with spindle and clear cells in hyalinized and fibroblastic stroma (B). Also, the tumor shows various growth pattern including solid sheet composed of epithelioid cells with high-grade cytologic atypia and mitosis (C), spindle cells (D), plasmacytoid cells (E), and clear cells (F).
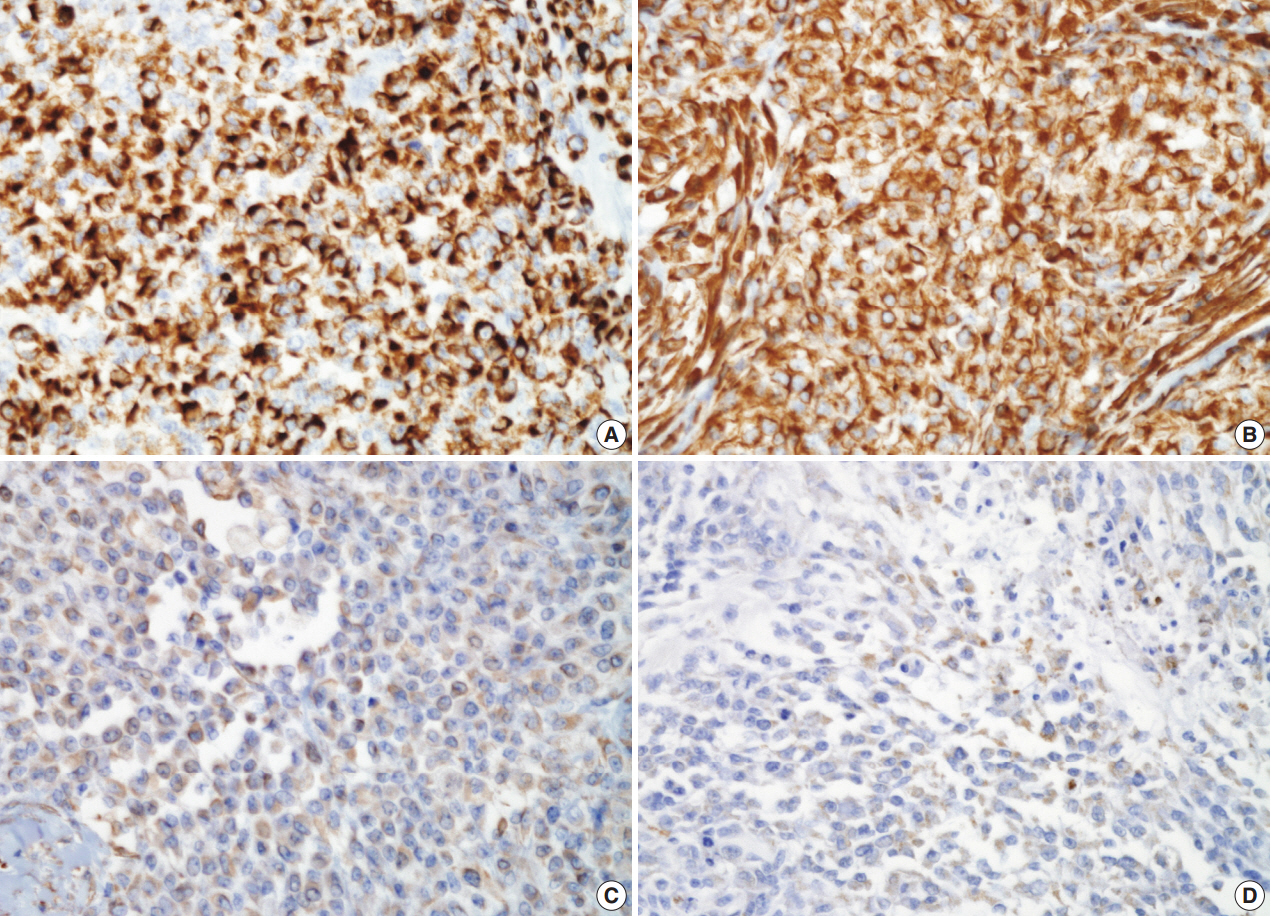
Almost all tumor cells were intensely positive for low-molecular-weight cytokeratin (CAM5.2), vimentin, and CD10. While the tumor cells were focally positive for cytokeratin and smooth muscle actin (SMA), they were diffusely weak positive for glial fibrillary acidic protein (GFAP). However, the tumor cells were totally negative for desmin, epithelial membrane antigen (EMA), CD15, monoclonal carcinoembryonic antigen, p63, CD34, human melanoma black 45, gross cystic disease fluid protein 15, and S-100 protein (Fig. 3). Fluorescence in situ hybridization (FISH) analysis for EWSR1 gene rearrangement was performed at an outside institution, but the present case did not exhibit EWSR1 gene rearrangement. On the basis of histologic findings and immunohistochemical study results, a diagnosis of myoepithelial carcinoma was rendered.
DISCUSSION
Myoepithelial carcinoma of soft tissue is morphologically identical to that of salivary gland. Although myoepithelial carcinoma in the salivary gland is a well-known tumor, only few cases of myoepithelial carcinoma of soft tissue have ever been reported because of their rarity. Myoepithelial carcinoma of soft tissue has not been reported in Korea or China. Only one case of myoepithelial carcinoma arising in the deep soft tissue of the forearm was reported from Japan [5]. The present case is the first report of myoepithelial carcinoma of soft tissue in Korea and the second one in East Asia.
To confirm the myoepithelial differentiation of the tumor, we used immunohistochemical staining with keratin, EMA, S-100 protein, GFAP, SMA, and vimentin as the immunohistochemical criteria, which have been used by other authors [1,2]. In our case, the tumor cells were positive for vimentin, CAM5.2, cytokeratin, SMA, and GFAP, but negative for EMA, p63, and S-100 protein. In fact, previous studies have revealed that the positivity of these markers was variable [1,2]. By comprehensive consideration of histologic features and immunohistochemical profiles of our case, the tumor is consistent with myoepithelial carcinoma.
Several pathologic criteria have been suggested for distinguishing myoepithelial carcinoma from benign myoepithelial tumor in soft tissue [1-5]. Like their counterparts in the salivary gland, cytologic atypia, high mitotic rate, infiltrative growth into surrounding soft tissues, and/or tumor necrosis have been adopted as criteria for myoepithelial carcinoma of soft tissue. Hornick and Fletcher [2] revealed that the microscopically infiltrative growth without cytologic atypia and mitotic activity was not associated with recurrence or metastasis. They emphasized that the cytologic atypia including prominent nucleoli, vesicular or coarse chromatin, and nuclear pleomorphism was suggestive of aggressive behavior and tendency for metastasis. Therefore, myoepithelial tumor of the soft tissue with at least moderate cytological atypia should be managed as myoepithelial carcinoma. In fact, there is no definitive cutoff of mitotic rate for malignant criteria.
EWSR1 is located on chromosome band 22q12 and encodes a promoter-specific transactivator. EWSR1 gene rearrangement is associated with several neoplasms such as Ewing sarcoma, desmoplastic small round cell tumor, clear cell sarcoma, angiomatoid fibrous histiocytoma, myxoid liposarcoma, and extraskeletal myxoid chondrosarcoma [4]. Also EWSR1 gene rearrangement has been found in myoepithelial tumors including myoepithelial carcinoma [1,4,6-8]. The EWSR1-ZNF444 fusion gene was found in a case of myoepithelial carcinoma of soft tissue with a lung metastasis [7]. Furthermore, a case of myoepithelial carcinoma of soft tissue with round cell morphology was positive for EWSR1 gene rearrangement on FISH analysis [8]. However, these reports do not mean that all myoepithelial carcinomas of soft tissue exhibit EWSR1 gene rearrangement. Gleason and Fletcher1 described two cases with EWSR1 gene rearrangement and one case without this aberration. Furthermore, EWSR1 gene rearrangements were observed in only 50% of myoepithelial carcinomas of soft tissue [4]. Our case did not have EWSR1 gene rearrangement by FISH analysis. Therefore, EWSR1 gene rearrangement may not be generally present in myoepithelial carcinoma of soft tissue. Recently, a case of myoepithelial carcinoma of soft tissue without EWSR1 gene rearrangement showed multiple metastases leading to patient death [6], suggesting the need for a careful follow-up in our case.
The heterogeneity of immunophenotype and various histologic patterns and cell types of myoepithelial carcinomas of soft tissue make the differential diagnoses broad, including metastatic carcinoma, malignant melanoma, proximal-type epithelioid sarcoma (ES), extraskeletal myxoid chondrosarcoma (EMC), epithelioid malignant peripheral nerve sheath tumor (MPNST), and poorly differentiated synovial sarcoma. Immunohistochemistry is absolutely important for diagnosis of myoepithelial carcinoma of soft tissue because of its morphological similarity to other neoplasms.
Metastatic carcinoma usually has the clinical history of old age and a primary tumor arising in another location. In contrast to myoepithelial carcinoma, metastatic carcinoma usually lacks the myxoid stroma and distinct multinodular growth pattern [2]. Furthermore metastatic carcinoma is negative for S-100 protein, GFAP, and myogenic markers. Malignant melanoma can be excluded by the rarity of expression of GFAP, cytokeratin, and myogenic markers: myxoid stroma is also rare in malignant melanoma [2].
Proximal-type ES often shows a multinodular growth pattern, consisting of large epithelioid carcinoma-like cells with vesicular nuclei and prominent nucleoli. Rhabdoid features are frequently observed in ES. However, cytomorphologic uniformity with sheet-like growth pattern is characteristic in ES in contrast to the heterogeneous cytomorphology and myxoid stroma in myoepithelial carcinoma [9]. Moreover, ES is negative for S-100 protein, GFAP, and myogenic markers [2].
EMC is also a differential diagnosis of myoepithelial carcinoma of soft tissue. The rhabdoid feature of high-grade EMC mimics the plasmacytoid morphology of myoepithelial carcinomas. However, EMC has a distinct lobular pattern with uniform myxoid change and lacks expression of epithelial, myogenic markers, and GFAP [2].
Epithelioid MPNST can resemble myoepithelial carcinoma due to its lobular architecture with myxoid stroma composed of epithelioid tumor cells and S-100 protein and/or GFAP expression. However, epithelioid MPNST shows more architectural and cytomorphologic uniformity than myoepithelial carcinoma and lacks expression of epithelial, myogenic markers and GFAP [1].
Poorly differentiated synovial sarcoma lacks the characteristic features of myoepithelial carcinoma including lobular architecture, epithelioid cells and expression of myogenic markers and GFAP [10].
In summary, we are reporting the first case of myoepithelial carcinoma of soft tissue in Korea. To the best of our knowledge, the present case is the second report from East Asia described in the English literature. Myoepithelial carcinoma should be considered in the differential diagnosis of malignant tumors that have various morphologic features of tumor cells including epithelioid, spindled, plasmacytoid, or clear cell with myxoid background in soft tissue. Although further study is needed to confirm the criteria for myoepithelial carcinoma of soft tissue, pathologists have to be conscious that myoepithelial tumors of soft tissue with cytologic atypia, mitotic activity, and tumor infiltration suggest the malignant potential of myoepithelial tumor of soft tissue.
Notes
No potential conflict of interest relevant to this article was reported.
Acknowledgements
This work was supported by Inha University Research Grant.