TdT+ T-Lymphoblastic Proliferation in Castleman Disease
Article information
The concept of indolent T-lymphoblastic proliferation (iT-LBP) was introduced by Velankar et al. [1] as the proliferation of immature terminal deoxynucleotidyl transferase (TdT)+ T cells in extrathymic tissue without the involvement of bone marrow or peripheral blood [2]. Although the histopathologic features and the immunophenotype are similar to T-lymphoblastic lymphoma (T-LBL), iT-LBP is a benign proliferation of thymocytes that requires no treatment [1-3].
Here, we report a case of iT-LBP in association with hyaline vascular-type Castleman disease in the retroperitoneum of a Korean female. A 37-year-old woman presented with right lower quadrant pain. Abdominal computed tomography scan demonstrated a well-defined homogeneous enhancing mass in the retroperitoneum (Fig. 1A). Laparotomy was performed and the mass was resected. Grossly, the resected mass (6.3×4.8×3.1 cm) was well-demarcated, round, and rubbery-firm. The cut surface was pinkish-yellow and fleshy, exhibiting central fibrosis (Fig. 1B). Microscopically, there was a small amount of proliferation of follicles of various shapes and sizes, and expansion of the interfollicular regions with sinus obliteration (Fig. 1C). Follicles demonstrated expanded mantle zones with onion skinning, regressed germinal centers with hyperplastic follicular dendritic cells reactive for CD21, radially penetrating blood vessels, and few follicle center cells (Fig. 1D-F). Many follicles contained more than one germinal center. The interfollicular region was filled with hyperplastic high endothelial venules, hyalinizing fibrosis, and an admixture of plasma cells, eosinophils, immunoblasts, plasmacytoid dendritic cells, and lymphocytes. In addition, there were multiple nodular and diffuse areas of lymphoid cell infiltration in the interfollicular and perifollicular regions, which were composed of small- to medium-sized cells with a high nuclear/cytoplasmic ratio and slightly irregular nuclei with open chromatin and inconspicuous nucleoli (Fig. 2A, B). These cells were immunoreactive for TdT, CD3, CD4, and CD8, but negative for CD20 (Fig. 2C, D). The Ki-67 labeling index was also high (Fig. 2F). Some of the large cells in the interfollicular and perifollicular area appeared similar to dysplastic follicular dendritic cells; consequently, follicular dendritic cell sarcoma was considered. However, these cells were negative for CD21, CD23, or CD35. Systemic work-up revealed no evidence of lymphoma involvement. Polymerase chain reaction study of the T-cell receptor gamma genes using BIOMED-2 primers (Invivoscribe, San Diego, CA, USA) failed to produce evidence of monoclonal rearrangement (Fig. 2G). A provisional diagnosis of iT-LBP associated with hyaline vascular-type Castleman disease was established and the patient was observed without any further treatment. On three-month follow-up, the patient was free from disease.
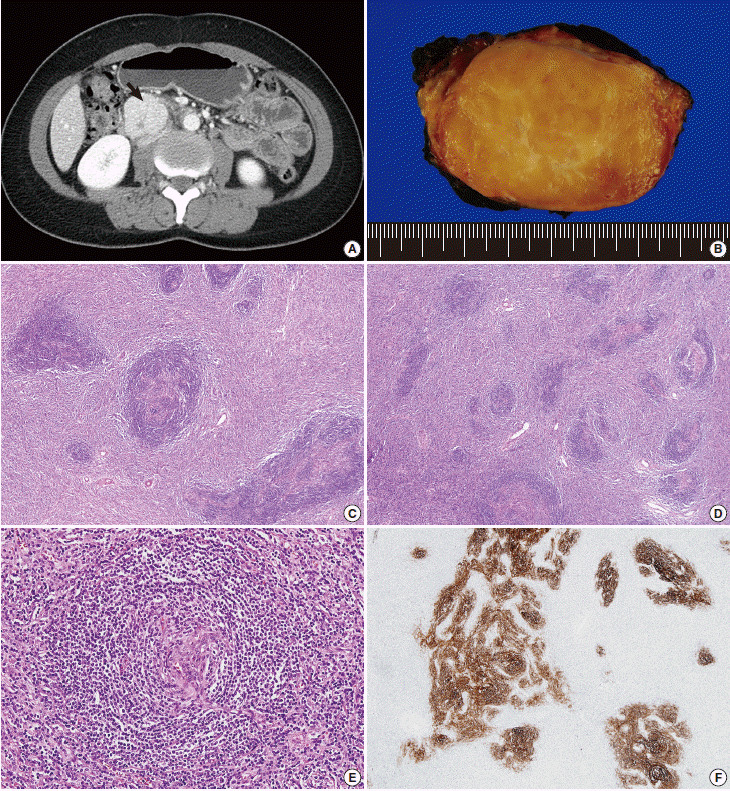
Hyaline vascular-type Castleman disease. (A) Abdominal computed tomography demonstrates a well-defined homogeneous enhancing mass (arrow) in the retroperitoneum. (B) The mass exhibits a fleshy cut surface with central fibrosis. (C) Variably-sized follicles with multiple germinal centers are present. The interfollicular compartment is expanded with vascular proliferation. (D) Follicular structures appear smaller due to the regression of germinal centers. (E) Mantle zone lymphocytes have a laminated appearance, similar to onion skin. The vessel penetrating the atrophic follicle has a lollipop-like appearance. (F) Hyperplastic follicular dendritic cells are stained with CD21.
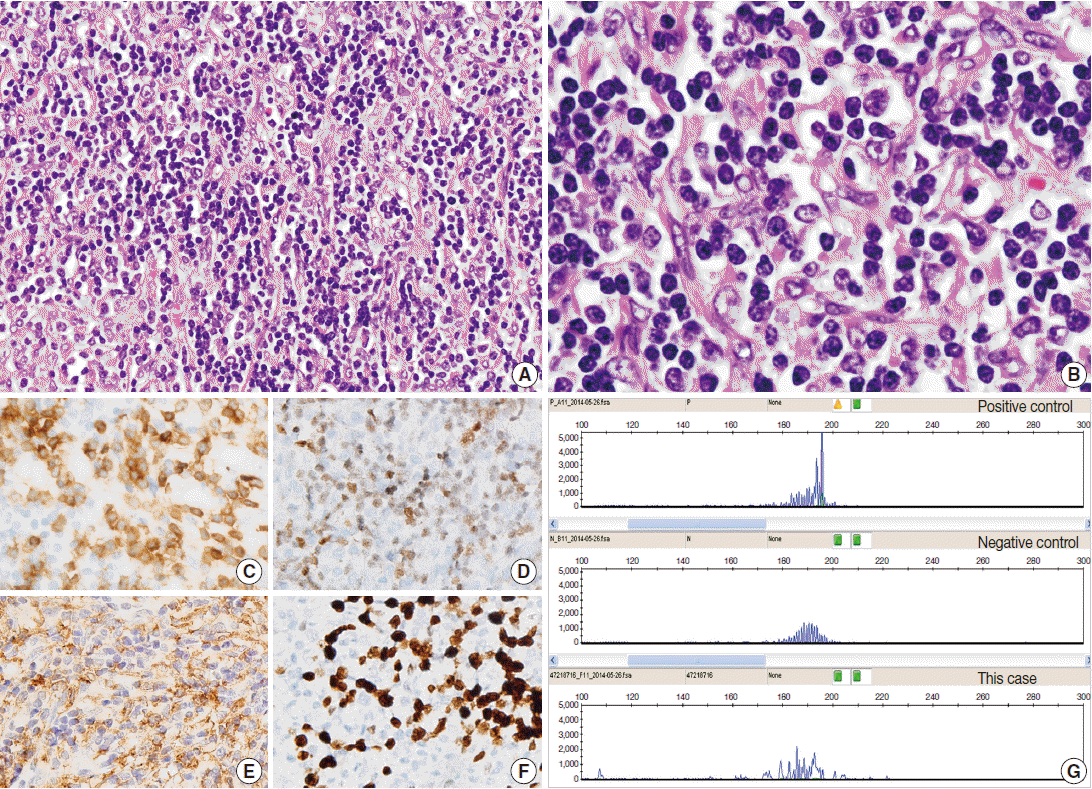
Indolent T-lymphoblastic proliferation (iTLBP). (A) Nodular and diffuse areas of monotonous lymphoid cell infiltration in the interfollicular and perifollicular regions. (B) The cells show blastic nuclei with open chromatin and inconspicuous nuclei. (C–F) Cells are immunopositive for CD3 (C), terminal deoxynucleotidyl transferase (D), and CD33 (E), and have an increased Ki-67 labeling index (F). (G) Polymerase chain reaction study of the T-cell receptor gamma genes using BIOMED-2 primers shows no evidence of monoclonality.
A total of ten cases of iT-LBP associated with Castleman disease have been reported in the literature, with or without follicular dendritic cell tumor (FDCT) and various caricinomas. iT-LBP most commonly involves the lymph nodes, where it invades interfollicular/parafollicular areas without effacement of the architecture [2-5]. The cells are small to medium sized with blastic chromatin, and exhibit frequent mitotic figures without significant atypia or prominent nucleoli. The typical immunophenotype involves immature cortical thymocytes, expression of CD3 and TdT, coexpression of CD4 and CD8, and variable expression of other T-cell antigens (CD2, CD5, and CD7). iT-LBP may also exhibit CD10, CD99, and CD1a expression, but lacks CD34 or B-cell markers. The Ki-67 labeling index is high. The possibility of ectopic thymic tissue can be ruled out by the absence of cytokeratin-staining epithelial cells. Ohgami et al. [3] reported that the presence of TdT-positive T cells is not rare in lymph nodes with Castleman disease, FDCT, and angioimmunoblastic T-cell lymphoma, and that these cells are rarely noticeable on hematoxylin and eosin sections, although they are usually present at low density in a scattered distribution. Occasionally, they will form dense patches or tumors, and may mimic T-LBL with TdT positivity and a high Ki-67 labeling index. In contrast to T-LBL, which is a malignant monoclonal process that may involve peripheral blood, bone marrow, and the thymus, with tissue effacement, iT-LBP does not involve peripheral blood, bone marrow, or the thymus. In lymph nodes, iT-LBP does not lead to the tissue effacement typical of lymphomatous growth. A recent study indicated that iT-LBP could involve multiple lymph nodes and show partial CD33 expression [5]. In this case, immunopositivity for CD33 was also observed (Fig. 2E); however, node effacement as absent and the structure was preserved.
How immature lymphocytes localize and proliferate outside the context of thymic epithelial support remains unclear [3,4]. We suppose that immature T cells released from the thymus travel via the peripheral blood and eventually reach the lymph nodes. In a state of immune system dysfunction, these immature cells may proliferate like mature lymphocytes.
It is essential that clinicians are aware of this entity in order to avoid a misdiagnosis of T-LBL and consequent unnecessary treatment. Careful examination of morphologic features, immunochemical staining, molecular study, extent of involvement, and clinical course can contribute to the differentiation of iT-LBP from T-LBL.
Notes
Conflicts of Interest
No potential conflict of interest relevant to this article was reported.