An Extremely Rare Case of Back and Hip Pain due to the Metastasis of Late Recurrent Myxopapillary Ependymoma to the Inguinal Lymph Node
Article information
Myxopapillary ependymomas (ME) are rare and slowly growing gliomas usually with spinal cord localization originating from the ectopic ependymal residues. They are commonly located in the conus medullaris, cauda equina and filum terminale. ME is known to be more aggressive in childhood, but has a good prognosis in adults with a very low metastasis risk [1,2]. If successfully excised, ME are usually cured completely. However, in adults, ME at the sacrococcygeal region metastasizing to organs outside central nervous system has rarely been reported so far [3-5]. Herein, we report an extremely rare case of an inguinal lymph node metastasis developed 19 years after removal of primary ME at the sacrococcygeal region, which presented with unusually severe back and hip pain. Because the metastasis of ME to the lymph node after such a long time is rare, our case may contribute to improving the differential diagnosis of extremely rare metastatic ME cases and identifying their unexpected role in the pain of unknown origin.
CASE REPORT
A 36-year-old woman presented to our surgical clinic with severe back and hip pain that had been present for a year. She was otherwise healthy except for histories of initially named “cyst” operation at the sacrococcygeal region at 17 years of age and cesarean section delivery at 32 years of age. Left inguinal lymphadenopathy (around 3 cm in diameter) was noted. Magnetic resonance image (MRI) scanning of the spinal cord showed minimal fibrotic changes and edema in the posterior subcutaneous soft tissue at the distal tip of the coccyx, just to the right of the midline. These findings suggested that the lymphadenopathy might have developed as secondary to the previous sacrococcygeal cystic mass operation at 17 years of age. The inguinal lymph node was totally excised.
The macroscopic cut-section of the lymph node showed a near-total effacement of the normal structure with soft and grayish gelatinous appearance. Hematoxylin and eosin (H&E) staining revealed a metastatic tumor (Fig. 1A) showing cystic and papillary structures with a single-row cuboidal-columnar epithelium in the myxoid and vascularized stroma and mucinous material within the lumens (Fig. 1A–C). In addition, periodic acid-Schiff–Alcian blue (PAS-AB) histochemical staining confirmed the presence of acidic mucin (Fig. 2A).
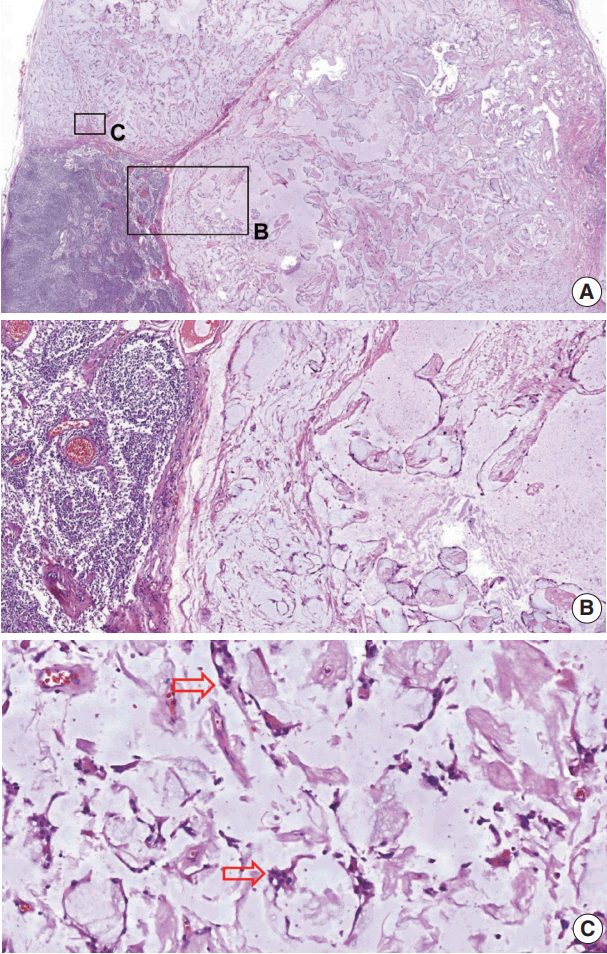
Metastatic tumor in the lymph node. (A) In the lower-left corner is a normal lymph node structure; in the remaining areais the metastasis. (B, C) Higher magnification view of the metastasiscystic and papillary structures (B) with a single-row cuboidal-columnar epithelium in the myxoid and vascularized stroma (arrows) (C). Lumens contain mucinous material.

Histopathological findings suggest that the tumor is originated from the central nervous system, consistent with myxopapillary ependymoma metastasis. (A) Periodic acid-Shiff–Alcian blue histochemical stain shows the presence of acidic mucin. (B) Epidermal growth factor receptor positivity of the tumor cells by immunohistochemistry (IHC). (C) Glial fibrillary acidic protein positivity of the tumor cells by IHC. (D) Vimentin positivity of the tumor cells by IHC.
A wide range of immunohistochemical (IHC) staining panel was applied to cover epithelial and mesenchymal tumors, germ cell neoplasms and malignant mesothelioma. While the tumor was positively stained with vimentin, glial fibrillary acidic protein (GFAP), epidermal growth factor receptor (EGFR), and S100 (Fig. 2B–D and data not shown, respectively), there was no staining for pan-cytokeratin (5/6/8/18), CDX2, cytokeratin (CK) 20, CK7, α-fetoprotein, SALL4, epithelial membrane antigen, CD31, CD34, WT1, calretinin, CD117, CD10, OLI˙G2, p63, and MOC31. The Ki-67 labeling index was very low with only 1%–2%. The results of IHC stainings together with the history of the patient were evaluated, and we concluded that the case was compatible with inguinal lymph node metastasis of ME. Unfortunately, the original radiology and pathology materials were not available for review.
The institutional review board (IRB) approval has been waived, but informed consent was obtained from the patient (2016-12/05).
DISCUSSION
Myxopapillary ependymoma is most commonly seen in conus medullaris, cauda equina, and filum terminale. The subcutaneous, sacrococcygeal, or presacral-situated extramedullary MEs are considered as a prominent subgroup originating from the ectopic ependymal residue. Long-term leg pain is a typical clinical symptom of ME. Depending on its settlement location, a total removal of the tumor can be difficult, which may cause recurrence after years. It is very rarely found that ME may progress along the spinal nerves during its repetition and thus may cause multiple lesions in the spinal canal via cerebrospinal fluid [4].
ME is known to be more aggressive in childhood, but has a good prognosis in adults with a very low risk of metastasis [1,2]. According to the World Health Organization report in 2015, metastasis was detected in only 17 cases out of 183 cases, 11 of which involved brain metastasis [1]. All of these previously reported cases of young patients. In adults, sacrococcygeal placement of ME metastasis has been reported to be extremely rare and is often presented as soft tissue or bone metastasis [5,6].
In our case, the patient had a history of previous operation at the sacrococcygeal region 19 years prior with an initial coccygeal cyst. MRI findings of the left inguinal lymphadenopathy and minimal fibrotic changes and edema of the posterior subcutaneous soft tissue at the coccyx suggested ME metastasis. H&E sections of the lymph node confirmed the metastasis of myxoid and vascularized stroma showing cystic and papillary structures with single rowed cuboidal columnar epithelium and mucinous material within the lumen. Positive IHC staining for vimentin, GFAP, S100, and EGFR was observed. Negativity for cytokeratin markers and specific positivity for GFAP, vimentin, and S100 are especially noted in ME [2]. The presence of acidic mucin in myxoid matrices shown by PAS-AB histochemical staining is typical of ME,2 and we found similar positive staining with PAS-AB stain. In addition, Ki-67 proliferation index was very low (1%–2%) in our case, supporting the earlier literature [2,4]. Recent studies have also focused on EGFR positivity because EGFR positivity is an indicator of aggressive tumor progression [2]. These morphologic and IHC findings of our case are consistent with ME metastasis, even though the initial pathology of the primary tumor is not available. A recurrence of ME within 12–15 years has been reported. However, metastasis to the lymph nodes is very rare and only a few cases with late metastasis have been reported (Table 1) [5,7-10].
In conclusion, to the best of our knowledge, the current case is an extremely rare case of ME occurring at an adult age with lymph node metastasis after a long time. Thus, this case has multiple implications for future pathological diagnosis. First, in cases of lymph node metastasis presenting as innocuous myxopapillary tumor morphology, myxopapillary ependymoma should be additionally considered. In such cases, origin of the primary tumor such as conus medullaris, cauda equina and filum terminale should be investigated. Second, the rarity of our case sheds light on the fact that these tumors may metastasize after many years. The role of MRI in the diagnosis of ME is important but may not be conclusive by itself in cases like ours presenting as a lymph node metastasis. Therefore, examination of all past surgical records and pathology reports can aid in diagnosis. Lastly, our case may contribute to the understanding of the unexpected role of rare and late ME metastasis in the differential diagnosis of pain of unknown origin.
Notes
Conflicts of Interest
No potential conflict of interest relevant to this article was reported.
