Sudden Child Death due to Thrombotic Giant Coronary Artery Aneurysms Complicated by Atypical Kawasaki Disease: An Autopsy Case
Article information
Kawasaki disease (KD) is an acute, self-limited vasculitis with a specific predilection for the coronary arteries, and it occurs predominantly in infancy and early childhood. Coronary artery complications develop in up to 25% of affected children if the disease is left untreated [1]. Typical KD presents a high swinging fever (often > 39°C) that persists for 5 or more days despite treatment. However, the diagnosis of KD is difficult due to obscure clinical manifestations. The atypical or incomplete form of KD is most common in children younger than 6 months or older than 5 years, and diagnosis is important due to a higher incidence of coronary artery abnormalities [2]. In Korea, there has been limited information on postmortem findings in the pediatric population [3].
Here, we report an autopsy case of a 23-month-old child with a history of the atypical form of KD, who developed bilateral giant aneurysms of the coronary arteries.
CASE REPORT
A 23-month-old boy collapsed suddenly after taking a bath at home and was transferred to an emergency center. On arrival, he was unresponsive and expired despite intensive resuscitation. Six weeks previously, he had visited a local clinic with complaints of mild fever, general myalgia, and diarrhea. The pediatrician found conjunctival injection and a facial rash. However, oral mucosal changes, such as strawberry tongue and cervical lymphadenopathy, were not present. He was treated under the presumptive diagnosis of upper respiratory infection. A mild fever was persistent even under medical care. There were no specific complaints except that his activity had decreased for several days before the event.
At autopsy, no significant trauma or injury was noted. The patient was underweight at only 15 kg. The heart weighed 140 g, with a smooth epicardium. The pericardial space was filled with 55 mL of yellowish effusion. The right coronary artery (RCA) had three saccular aneurysms, occluded by thrombi, measuring 3.4 cm × 2.0 cm × 1.1 cm, 3.1 cm × 1.7 cm × 1.2 cm, and 0.7 cm × 0.3 cm × 0.3 cm, respectively. The left main coronary artery and the proximal part of the left anterior descending coronary artery (LAD) formed a bilocular aneurysm with a large thrombus, measuring 3.7 cm × 2.0 cm × 1.8 cm (Fig. 1). The left circumflex coronary artery was not dilated. Multiple dark, mottled areas were observed on a cross section of the myocardial wall. The affected area showed various morphologies depending on the duration after the infarction. The posterior wall of the left ventricle (LV) showed several yellow-tan infarct sites, the largest of which measured 1.5 cm × 1.0 cm. The anterior and posterior walls of the right ventricle (RV) showed gray, depressed infarct sites, or gray-white scar lesions, which indicated irreversible myocardial injury accordant to 10 to 14 days. Microscopically, foci of acute coagulative necrosis and contraction bands were seen in the posterior wall of the LV, which usually become detectable in the first four to 12 hours after injury (Fig. 2A). Polymorphonuclear leukocytes infiltrated around the necrotic muscle fibers. Some areas of the posterior wall of the LV showed hemosiderin-laden macrophages and fibrovascular granulation tissue. Histologic findings of the posterior wall of the LV demonstrated various morphologic spectrums, ranging from 1 to 14 days. The RV wall revealed granulation tissue with neovascularization and collagen deposition (Fig. 2B). The anterior wall of the LV and the interventricular septum were preserved. Microscopic examination of the LAD revealed mural lymphoplasmacytic infiltration, adventitial fibrosis, and neovascularization. The RCA showed polymorphonuclear leukocyte and lymphocyte infiltration with focal acute necrosis, which resulted in destruction of the internal elastic lamina (Fig. 3).
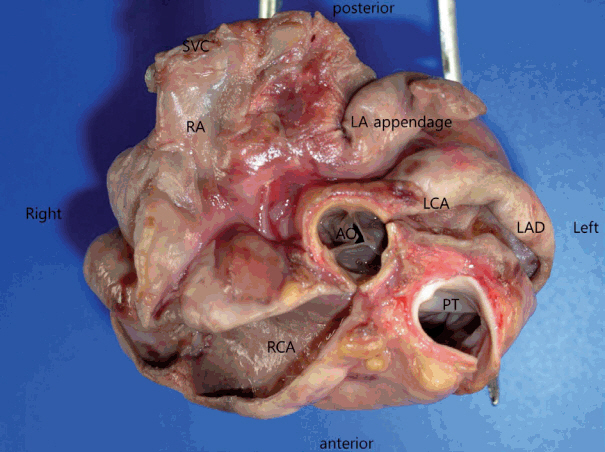
Coronary artery aneurysms. The left main coronary artery (LCA), left anterior descending coronary artery (LAD), and right coronary artery (RCA) form giant aneurysms. Luminal thrombi were removed during examination. AO, aorta; LA appendage, left atrium appendage; PT, pulmonary trunk; RA, right atrium; SVC, superior vena cava.
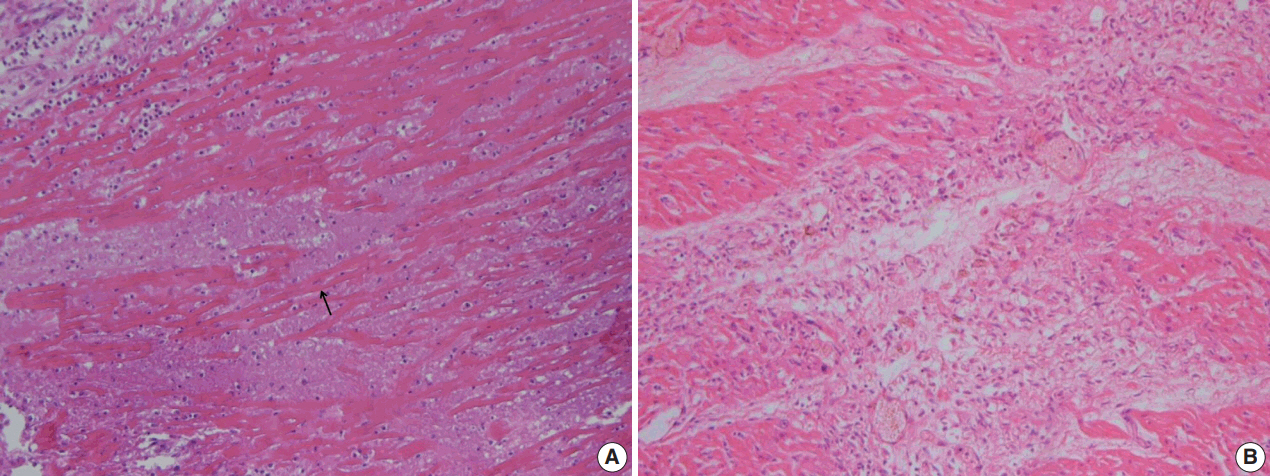
Myocardial infarction of ventricular wall. (A) Posterior wall of left ventricle shows coagulative necrosis along with wavy fibers (arrow). Widened spaces between the dead myofibers contain edema fluid and scattered neutrophils. (B) Posterior wall of right ventricle reveals granulation tissue characterized by loose collagen and capillaries. Patchy fibrosis is observed, with compensatory hypertrophic changes in adjacent myocytes.

Elastin stain. The right coronary artery shows destruction of internal elastic lamina and inflammatory cell infiltration.
The cause of death was associated with acute and chronic ischemic injury of the heart muscle due to thrombotic coronary artery aneurysm (CAA), complicated by atypical KD.
Written informed consent was not required with a waiver by the Institutional Review Board of Kyungpook National University (KNUH 2018-04-015).
DISCUSSION
KD is an acute inflammatory vasculitis of small- and mediumsized arteries [1]. It predominantly affects coronary arteries, resulting in weakening, aneurysm, and myocardial infarction. The diagnosis of KD is established by the presence of fever persisting for 5 days or more, along with at least four of the five principal symptoms as follows: (1) bilateral bulbar conjunctival injection without exudates; (2) changes in the lips and oral cavity; (3) changes in the extremities, such as edema or erythema of hands and feet, or desquamation of the fingertips; (4) polymorphous exanthema; and (5) cervical lymphadenopathy. According to a large-scale retrospective review of KD, the majority of patients were 1 to 4 years old (62%) [4]. In cases of children presenting fever and fewer than four of the other symptoms, diagnoses of “incomplete” or “atypical” KD were given. Atypical KD usually occurs in children younger than 6 months or older than 5 years [2]. Most reported cases of sudden unexpected death associated with incomplete or atypical KD were in infants less than 6 months of age [5,6]. The mortality peak for infants under 1-year-old occurs 15–45 days after the onset of symptoms [5]. In this case, death occurred one and a half months after the onset of symptoms, which was consistent with other cases. The diagnosis of KD in children is difficult because its presentation is usually similar to that of other viral or bacterial infections. In particular, atypical presentation may lead to delayed diagnosis and inappropriate therapy. In this case, the initial symptoms were obscure, without the typical manifestation of KD. First-line therapies for KD, such as aspirin and intravenous immunoglobulin, were not applied. In this case, the age at onset of atypical KD was older than usual, and the development of CAA progressed rapidly. The diagnosis was given at autopsy.
According to a review of studies about the distribution of CAA, the LAD is most commonly affected, followed by the RCA [7]. Characteristically, CAAs caused by KD are located in the proximal segments of coronary arteries. However, large CAAs (≥ 8 mm in diameter) were frequently found in the RCA rather than the LAD [7]. Aneurysm size is a major predictor for the development of myocardial infarction [7,8]. Larger CAAs indicated more extensive disease and an increased likelihood of bilateral CAAs. Furthermore, the outcome for patients with bilateral CAAs was worse than for patients with unilateral CAA [7]. In this case, there were bilateral giant coronary aneurysms involving the proximal LAD and the RCA. The pathologic features of cardiac complications from KD are rarely reported. Coronary vasculitis is pathognomonic for KD. A recently proposed model of coronary arteriopathy revealed three pathological processes, which include necrotizing arteritis, subacute/chronic vasculitis and luminal myofibroblastic proliferation [8]. Coronary arteritis results in rapid destruction of luminal endothelial cells, elastic lamina and medial smooth muscle cells. This causes loss of integrity of the arterial wall, eventually resulting in aneurysm formation. Giant CAAs larger than 8 mm in diameter do not resolve, regress or remodel [8].
In adult patients, KD may remain clinically silent for decades after onset. On the other hand, atypical KD is more common in infants and poses a higher risk for developing CAA. KD continues to have a clinical diagnosis based on signs and symptoms without specific markers; this can result in diagnostic dilemmas, particularly in atypical cases. Atypical KD must be considered when prolonged fever is present in young infants or children who do not fulfill the classical KD diagnostic criteria. Supplemental laboratory findings, such as elevated erythrocyte sedimentation rate and C-reactive protein, and echocardiographic criteria, are recommended for evaluation of suspected atypical KD.
Herein, we report a case of atypical KD with bilateral giant aneurysms in the LAD and the RCA. This case, which occurred in a 23-month-old boy, developed at an unusual age and rapidly progressed to CAA, leading to death. The pathologic features of myocardial infarction can provide information to better understand the progression and severity of CAA.
Notes
Conflicts of Interest
No potential conflict of interest relevant to this article was reported.
