2019 Practice guidelines for thyroid core needle biopsy: a report of the Clinical Practice Guidelines Development Committee of the Korean Thyroid Association
Article information
Abstract
Ultrasound-guided core needle biopsy (CNB) has been increasingly used for the pre-operative diagnosis of thyroid nodules. Since the Korean Society of the Thyroid Radiology published the ‘Consensus Statement and Recommendations for Thyroid CNB’ in 2017 and the Korean Endocrine Pathology Thyroid CNB Study Group published ‘Pathology Reporting of Thyroid Core Needle Biopsy’ in 2015, advances have occurred rapidly not only in the management guidelines for thyroid nodules but also in the diagnostic terminology and classification schemes. The Clinical Practice Guidelines Development Committee of the Korean Thyroid Association (KTA) reviewed publications on thyroid CNB from 1995 to September 2019 and updated the recommendations and statements for the diagnosis and management of thyroid nodules using CNB. Recommendations for the resolution of clinical controversies regarding the use of CNB were based on expert opinion. These practical guidelines include recommendations and statements regarding indications for CNB, patient preparation, CNB technique, biopsy-related complications, biopsy specimen preparation and processing, and pathology interpretation and reporting of thyroid CNB.
Ultrasound (US)-guided fine-needle aspiration (FNA) is a cost-effective and safe tool for the assessment of thyroid nodules and has been used as a standard diagnostic modality for the management of thyroid nodules [1,2]. In the 1980s, FNA became the standard diagnostic tool for thyroid nodules, replacing large-needle biopsy because of its high diagnostic accuracy and low complication rate [3]. The main clinical role of FNA is to rule out malignant tumors requiring surgery. The use of FNA has reduced the number of unnecessary surgeries in patients with thyroid nodules. Although FNA is associated with high diagnostic accuracy and safety, it has several limitations due to a substantial rate (approximately 22.4%) of inconclusive results including non-diagnostic or atypia of undetermined significance (AUS)/follicular lesion of undetermined significance (FLUS) [4]. The non-diagnostic rate in initial FNA is about 10% and an even higher rate of up to 50% in repeat FNA [5,6]. The rate of AUS/FLUS is about 10%–20% with higher rates of inconclusive results in repeat FNA [7-10]. FNA also shows a relatively low diagnostic accuracy for follicular lesions [11,12]. These limitations associated with FNA lead to repeated FNA or unnecessary surgeries. Therefore, additional diagnostic tools for thyroid nodules are desirable to overcome the limitations of FNA for thyroid nodule assessment.
Advances in core needle biopsy (CNB) have led to the use of spring-activated single- or double-action needles in thyroid nodule diagnosis. In addition, widespread use of high-resolution US enables accurate diagnosis and minimization of complications associated with CNB in head and neck lesions [13]. CNB is safe, well-tolerated, and associated with a low incidence of complications when performed by an expert [1,14]. Several large-scale studies [15,16] and a systematic review and meta-analysis [17] validated the low rates of major and minor complications and absence of procedure-related deaths.
CNB has the potential to overcome the limitations of FNA by obtaining a large tissue sample, which reduces non-diagnostic results due to paucity of follicular cells and provides further information related to histological architecture underlying the capsule. Previous studies [18-21] reported that CNB yielded a lower rate of inconclusive results, including non-diagnostic or AUS/FLUS results compared with repeated FNA in the assessment of nodules with prior inconclusive results. Recently, several studies also reported the potential role of CNB as a first-line diagnostic tool for the management of thyroid nodules [22-25].
Together with thyroid FNA and CNB, a multidisciplinary approach for patients with a thyroid nodule is essential to improve the quality of life and achieve a better outcome. Standardized guidelines are needed to ensure the optimal management of patients with thyroid nodules. The terminology for reporting thyroid FNA cytology worldwide is based on the Bethesda System for Reporting Thyroid Cytopathology (TBSRTC), which was introduced in 2007. The second edition of TBSRTC was released in 2017 [26]. The Korean Society of Thyroid Radiology (KSThR) published ‘Core needle biopsy of thyroid nodules: consensus statement and recommendations’ in 2013 [27]. The Korean Endocrine Pathology Thyroid CNB Study Group published an initial proposal of ‘Pathology Reporting of Thyroid Core Needle Biopsy’ in 2015 [28]. In 2017, the KSThR published ‘Core Needle Biopsy of the Thyroid: 2016 Consensus Statement and Recommendations from the Korean Society of Thyroid Radiology’ [14]. The fourth edition of the World Health Organization (WHO) classification of endocrine tumors was released in 2017 [29]. The 2017 revisions of TBSRTC and the WHO classification in thyroid cytology and histopathology encompass molecular diagnostics and new diagnostic entities of borderline thyroid tumors such as noninvasive follicular thyroid neoplasm with papillary-like nuclear features (NIFTP).
Along with recent advances in the diagnostic classification and management guidelines of thyroid nodules, the Practice Guideline Committee of the Korean Thyroid Association (KTA) organized a taskforce for the development of practical guidelines of CNB for the diagnosis and management of thyroid nodules. This guideline includes the indications, patient preparation, biopsy technique, complications, specimen preparation, and pathology reporting.
METHODS
A search of the PubMed, MEDLINE, and EMBASE databases was performed to retrieve publications on thyroid CNB from 1995 to September 2019 using the following keywords: (thyroid nodule OR thyroid cancer OR thyroid carcinoma OR thyroid malignancy OR thyroid neoplasm OR thyroid diagnosis) and (core needle biopsy OR core-needle biopsy OR core biopsy OR gun biopsy OR CNB). Since clinical controversies related to issues exist in many of the areas, the recommendations regarding some of the issues are based on expert opinion. This limitation needs to be overcome in the near future when new elements of evidence are accumulated. Because there is little high-level evidence, recommendations regarding some of the issues are based on expert opinion. This limitation needs to be addressed in the future based on further studies. The goal of this guideline is to provide the best scientific evidence available and a consensus expert opinion regarding the clinical use of CNB in thyroid nodules.
INDICATIONS FOR CORE NEEDLE BIOPSY
The indications for CNB have yet to be clearly defined. Most thyroid guidelines recommend FNA as a first-line biopsy for thyroid nodule assessment; therefore, CNB is regarded as a complementary tool [14,30-32]. Recently, KSThR published the CNB Guideline in 2017 [14]. KSThR suggested several indications and the possible use of CNB for thyroid nodules with non-diagnostic or indeterminate FNA results. The National Cancer Institute (NCI) suggested that CNB under US guidance utilizing modern needles may be advantageous in cases rendered “unsatisfactory” by FNA [32]. In addition, the American Association of Clinical Endocrinologists (AACE)/American College of Endocrinology (ACE)/Associazione Medici Endocrinologi (AME), British Thyroid Association (BTA), and KSThR suggested the use of CNB for malignant thyroid tumors such as lymphoma, anaplastic cancer, medullary cancer, and metastasis [14,30,33]. However, the American Thyroid Association (ATA) did not recommend the use of CNB for thyroid tumors [31].
A meta-analysis showed that the non-diagnostic and inconclusive rates of CNB were 5.5% (95% confidence interval [CI], 2.2% to 8.7%) and 8.0% (95% CI, 4.4% to 11.5%), respectively, whereas the non-diagnostic and inconclusive rates of FNA were 22.6% (95% CI, 12.2% to 33.0%) and 40.2% (95% CI, 25.1% to 55.3%), respectively [34-36]. In another meta-analysis focusing on initially non-diagnostic FNA results, the non-diagnostic rate (6.4%; 95% CI, 3.3% to 16.1%) of follow-up CNB was significantly lower than that of repeated FNA (36.5%; 95% CI, 29.9% to 43.1%) [35,36]. In large cohort CNB studies, the false-negative rate of CNB ranged from 1% to 3% [35,37-39]. Recently, several studies suggested the initial use of CNB, but it is still disputed [23,37,38,40].
Based on current evidence, CNB has been suggested as the next diagnostic method for previously non-diagnostic FNA results [18,19,41,42], previous AUS/FLUS [18,43-45], and clinically suspected rare thyroid malignancies [13,46-49].
PREPARING PATIENTS FOR CORE NEEDLE BIOPSY
First, informed consent should be obtained and should include the information needed for the CNB procedure and possible complications [14]. The side effects associated with the use of drugs (i.e. bleeding tendency with drugs such as warfarin, heparin, aspirin, or clopidogrel bisulfate) should be evaluated, however, a screening blood test for coagulation is usually unnecessary. The KSThR guideline recommends withdrawal of aspirin and clopidogrel bisulfate for 7–10 days, warfarin for 3–5 days, and heparin for 4–6 hours before CNB [14]. Initiation of aspirin and clopidogrel bisulfate after CNB is recommended from the next day followed by warfarin at night, and heparin 2 hours later [14,50]. Considering the clinical significance of anticoagulant therapy, the withdrawal of anticoagulant should be carefully discussed with the prescribing physician. Warfarin can be transiently changed to shorter-acting heparin [14,50]. Fasting is usually not recommended for CNB in most patients [14].
PLANNING THE CORE NEEDLE BIOPSY PROCEDURE
Before the procedure, the nodule characteristics, size, location, and vascularity should be evaluated on gray-scale US and color Doppler US. Careful monitoring of vascularity with color Doppler US can minimize bleeding during CNB [14]. The CNB approach route is decided based on the information obtained from the pre-procedural US. Among the four approach routes available including transisthmic, lateral, longitudinal, and oblique [14], the trans-isthmic approach is considered the most suitable. The nodule size and location are important factors in deciding the size of specimen notch [14,18,39]. To improve the safety and diagnostic accuracy, CNB should be performed by experienced operators under US guidance [14].
PREPARATION OF CORE BIOPSY EQUIPMENT
Before 2000, large-bore needles (14-gauge) had been used for large-needle biopsy [14,51]. Recent CNB devices are characterized by small bore (usually 18–21 gauge) and spring activation [14,18,51-53].
The KSThR recommends appropriate CNB needle conditions for thyroid nodules [14]. First, a short needle length (less than 10 cm) is recommended since thyroid gland is a superficial organ. Second, needle bore determines the thickness of the specimen. The thinner the needle, the less damage to normal tissue but the lower is the amount of tissue obtained [14]. Some studies report the use of 16–22 gauge needles [18,41,53-55]. Although the use of 18–21 gauge needles is universal for thyroid nodules, 18-gauge needles have been mainly used in Korea [14,18,43,56-58]. Finally, the stylet length or the penetration length can be selected according to the nodule size. Thickness of core needle is another issue. Ahn et al. [59] reported that CNB with an 18-gauge needle is more effective for the diagnosis of thyroid nodules than CNB with a 20-gauge needle. However, there is insufficient evidence supporting the relationship between needle thickness and complication rate or diagnostic accuracy.
CNB needles are composed of two needles, the stylet and the cutting cannula [14,18,52]. The stylet (inner needle) carries an approximately 2-mm-long tip with a sharp slope to penetrate tissue and a specimen notch to hold the sampled tissue. The cutting cannula (outer needle) is the outer component for cutting the tissue [14].
Core biopsy devices are divided into automated and semi-automated types according to the mechanism of action [14]. The automated needle is known as the double-action device because both inner and outer needles are activated by spring action. This type of needle fires the inner needle by the action of a spring that can more easily penetrate hard fibrotic or calcified tissue. However, it may be more prone to tissue or vessel damage. The semi-automated needle is known as a single-action needle because the spring activates only once: the inner needle is manually advanced, followed by the spring-activated outer cutting needle. The semi-automated needle is relatively safe, since operators manually push the inner needle into the tissue [14]. The amount of tissue obtained depends on the needle thickness and the length of the specimen notch [35,60].
SAMPLING TECHNIQUES FOR CORE NEEDLE BIOPSY
Two options for needle guidance are available during US-guided CNB: a free-hand and a needle device under US guidance [29]. The KSThR Guideline recommends the free-hand technique (especially for experts) because it allows greater freedom for the operators in selecting the puncture point and adjusting the route during the procedure [14].
Patients lie down in a supine position under local anesthesia along the approach route. After vessel mapping using color Doppler US, a snapping wrist movement is favored for rapid and effective needle passage through the skin and thyroid capsule [14]. The entire length of the needle must be monitored during the procedure. Complete vessel mapping along the approach route (from the skin to the nodule) is crucial to avoid vessel injury and ensure safety of the procedure [14].
The number of core specimens is debatable. Hahn et al. [61] suggested at least two core specimens including a nodular tissue and a capsule for nodules with previous inconclusive cytology. The length of the core specimen is also important in that a large length decreases the biopsy number [38,62]. Suh et al. [38] performed less than two core biopsies; however, inconclusive results were not high in their factor analysis. Multiple biopsies can trigger further complications, which should be considered during CNB procedures [38].
Most importantly, the entire length of the needle and needle tip should be ensured during the CNB procedure [14,63]. The needle tip must be within thyroid capsule without large vessels along the imagined firing route. Further, the stylet can be fired followed by the cutting cannula (Fig. 1). When a successful method is used, the location of the specimen notch can be adjusted after stylet firing to select the most appropriate sampling site. Many studies suggest that the nodule tissue, nodule-parenchyma border (and/or visible capsule), and normal thyroid parenchyma should be obtained [14,54,60,64,65]. Nodules found in a dangerous location should be carefully evaluated. A single-action needle is safer because it enables fine adjustment of the specimen notch [14].
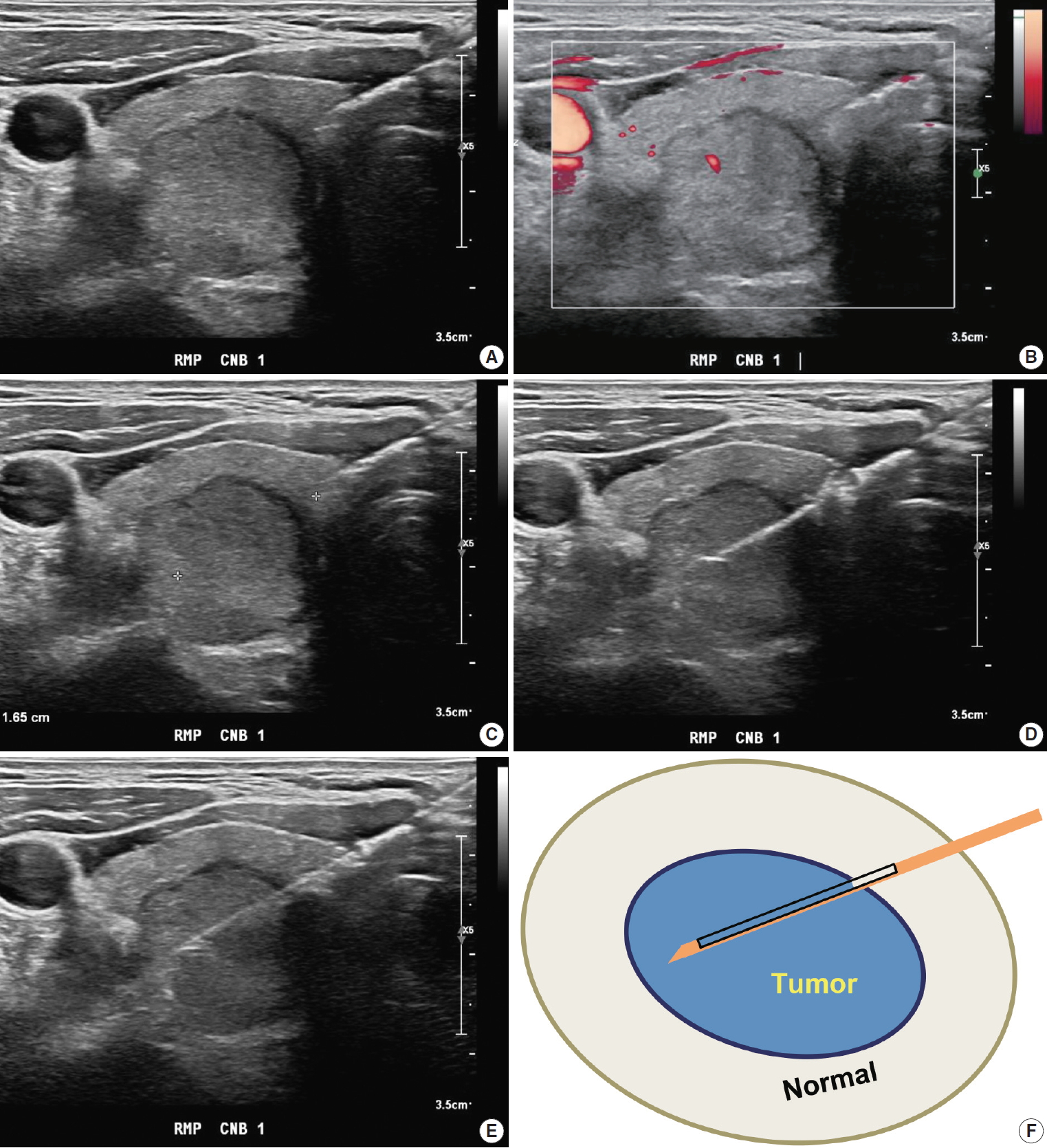
Core needle biopsy of right thyroid nodule. (A) Core needle approach is made through isthmus. (B) Vessels are evaluated with Doppler ultrasound advanced along the direction of the needle. (C) Measurement of distance of fire. (D, E) Firing the stylet first followed by cutting cannula. (F) Schematic illustration of core needle biopsy showing the biopsy site. A cylindrical core of tissue includes tumor and adjacent non-tumor tissue.
Occasionally, thyroid nodules exhibit severe fibrosis and/or hard calcifications. To successfully obtain samples from such hard nodules, using a double-action core needle is useful [14,66-69]. A guiding (coaxial) needle is useful for multiple CNB. In this technique, the biopsy needle is inserted through the lumen of the guiding needle, which is located from the skin close to the nodule surface. After CNB, the tissue sample can be visually assessed to determine if additional CNB is required [14,18,39,42,66]. After visual assessment, the harvested tissue should be immediately fixed in formalin. One to two biopsies are adequate for histological diagnosis. Manual compression should be performed immediately after the biopsy for 20 to 30 minutes [14].
BIOPSY-RELATED COMPLICATIONS
The KSThR or NCI guidelines suggest that CNB is safe, well-tolerated, and associated with a low incidence of complications when performed by experienced operators [14,18,32,41]. The reported complication rate is up to 4.1%, with the major complication rate up to 1.9% [16,43,53,55,58,70]. Various CNB complications were reported [14], including hematoma [18,39,41,53,70], voice change [43,55], infection [70,71], hemoptysis [41], edema [18,39,42], vasovagal reaction [70], and dysphagia [70]. A large single-center study (6,687 thyroid nodules of 6,169 patients) reported few major and minor complications (4/6,169 [0.06%] and 49/6,169 [0.79%], respectively) and no procedure-related death or sequelae [15]. A systematic review showed that the major complications including permanent voice changes, hematomas requiring hospital admission and a pseudoaneurysm, and minor complications were reported in six (0.04%) and 175 (1.18%) out of a total of 14,818 patients in 39 thyroid CNB studies from January 1994 to December 2016, respectively [17].
To avoid complications, CNB should be performed by an expert [14] with knowledge of anatomy, anatomic variations, and potential complications [72].
CNB is generally associated with pain and discomfort. US-guidance and the use of 18–21 gauge thin needles (commonly 18-gauge) can reduce the level of pain and the rate of complications [14]. Several studies compared FNA and CNB in terms of pain and tolerability and concluded that they were similar in both procedures [73-75]. A recent study reported that the overall satisfaction scores at 2 weeks after the procedure did not differ significantly between patients who underwent FNA and CNB [75].
Hematoma induced by vascular injury is the most common complication in thyroid CNB with a reported incidence of up to 3.9% [14,15,76], which is similar to that of FNA (1%–6.4%) [77]. Parenchymal edema induced by small vessel injury is frequently associated with hematoma and pain [18,39,42,66]. These vascular injuries are mostly managed with simple compression without the need for medication in most cases [14,18,39]. A few cases of hematoma have been reported after CNB as a major complication [53,78]. To prevent these complications, manual compression should be performed immediately after the biopsy for 20 to 30 minutes to prevent the risk of delayed hematoma [18,42].
Voice change, caused by direct injury or indirect injury due to bleeding or edema to the recurrent laryngeal nerve, is a rare but serious complication after thyroid CNB [37]. A systematic review showed that the overall incidence of permanent and transient voice change after thyroid CNB was 0.0013% (2/14,818) and 0.034% (5/14,818), respectively [17]. The highest incidence (1.9%, 1/54) of voice change was observed in a small study [43]. Recurrent laryngeal nerve palsy associated with hemorrhage/edema is usually transient [14,79]. A trans-isthmic approach under US monitoring is important to prevent direct injury to the recurrent laryngeal nerve [14,72]. A lateral-to-medial approach is dangerous to trachea, esophagus or recurrent laryngeal nerve [16].
Abscess formation is a very rare complication after CNB due to the rich vascular supply and lymphatic drainage, and high iodine content in the thyroid [14,71]. Therefore, the application of prophylactic antibiotics is not recommended before or after CNB.
In the case of a nodule located at the posteromedial margin of the thyroid, CNB is indicated after excluding a pharyngoesophageal or esophageal diverticulum [14,72,80]. Direct injury to the trachea induces cough or hemoptysis [41]. Tinnitus, a rare complication, has been attributed to injury of the perithyroidal vertebral artery and vein followed by an arteriovenous fistula [81]. Tumor seeding after CNB has not been reported but is a possible rare complication [82]. For safe and effective CNB procedure, operators should be familiar with the broad spectrum of complications and preventive methods [14].
PATHOLOGY REQUEST FORM
The request form for CNB should include the following information: identification of the patient sample: patient’s name, date of birth, unit number or medical record number; name of the requesting clinician and the clinician who performed the procedure; side and site of the lesion; number of biopsy cores; US imaging findings; and relevant clinical history.
PREPARATION AND PROCESSING OF CORE NEEDLE BIOPSY SPECIMENS
Proper tissue processing and histological staining procedures are essential for subsequent pathologic diagnosis. Biopsy specimens are fragile and should be handled with caution. As soon as the biopsy tissues are removed from the thyroid gland, biopsy cores should be wrapped in saline- or fixative-moistened gauze or filter paper to prevent specimen loss and tissue folding, and rapidly fixed with 10% neutral buffered formalin (NBF) (which is equivalent to 4% formaldehyde) to inhibit autolysis and putrefaction. Squeezing of biopsy specimen must be avoided to prevent cell rupture and loss of cell identity when examined microscopically. If a fixative cannot be added in a timely manner, the specimen should be wrapped in a moist saline gauze and refrigerated until the specimen is treated with an appropriate fixative. The ratio of formalin solution to tissue should be at least 10:1 with 10 mL of the fixative for every gram of tissue [83,84]. In clinical practice, the adequate volume of 10% NBF should be 15–20 times the specimen volume. The fixation time depends on sample size. Adequate fixation time with 10% NBF usually requires a minimum of 5 hours of exposure for small biopsy specimen [84]. However, practices may vary between laboratories. Incomplete or poor tissue fixation can lead to poor and unsatisfactory results. All specimens must be placed in a leak-proof container.
Sections obtained from formalin-fixed paraffin-embedded tissue blocks can be used for routine hematoxylin and eosin staining or ancillary tests such as special stain, immunohistochemistry, and molecular tests.
PATHOLOGY REPORTING FOR THYROID CORE NEEDLE BIOPSY
The first edition of ‘Pathology Reporting of Thyroid Core Needle Biopsy’ published in 2015 by the Korean Endocrine Pathology CNB Study Group has been widely used by Korean pathologists [28]. A recent study performed in China showed that the pathology reporting system of thyroid CNB is objective, operable and valuable for the pathologic diagnosis of thyroid nodules clinically [85].
The thyroid CNB pathology must be correlated with clinical and US imaging findings. The biopsy results should be reviewed in conjunction with matching surgical pathology after surgery.
A categorical reporting system for CNB ensures effective communication between pathologists and clinicians, with less likelihood of misinterpretation of pathology results. The six original general categories have been retained in the 2019 revision.
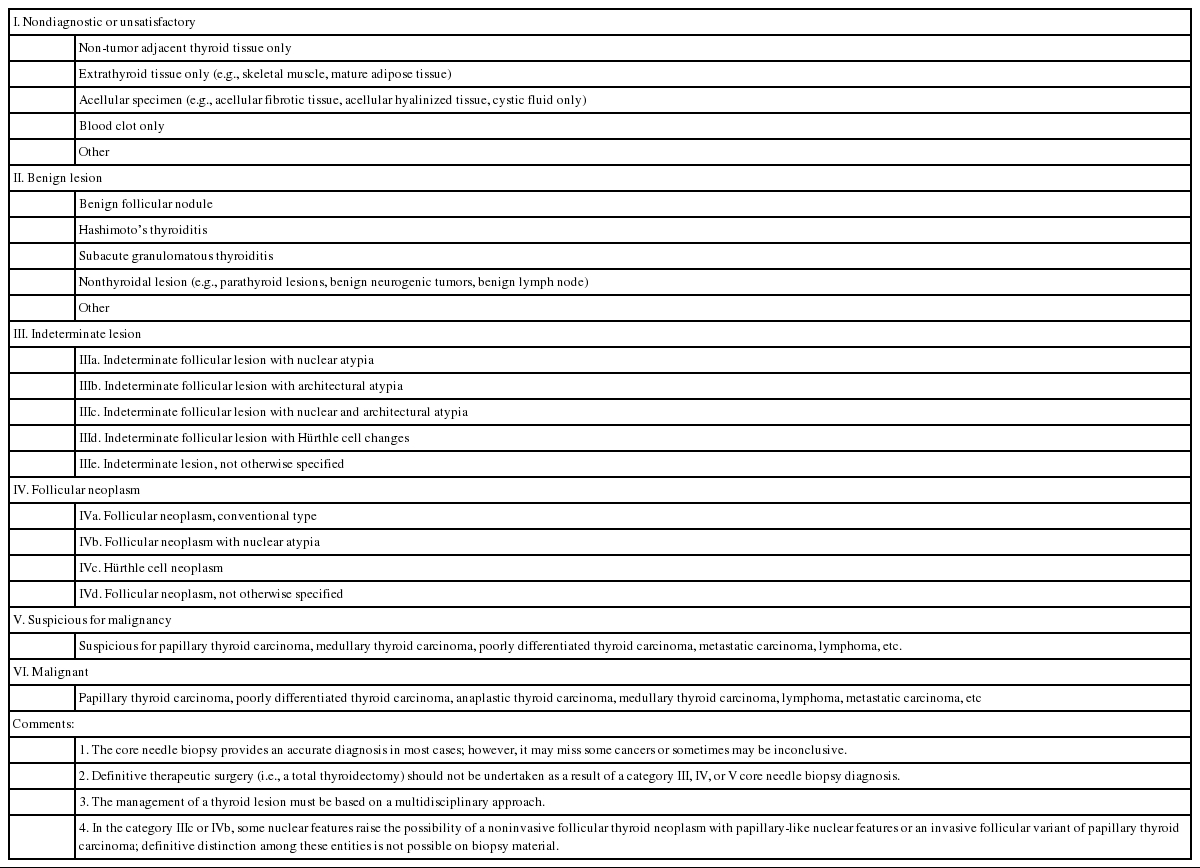
To communicate with clarity, the pathology report of thyroid CNB begins with a general diagnostic category. The six general diagnostic categories are shown in Table 1. Subcategorization is often informative especially in diagnostic categories III and IV. A brief microscopic description of the biopsy specimen can be informative, but should not be used alone to report the diagnosis. The standardized terms for the diagnostic categories should be used. The numerical code alone should not be used without the term of diagnostic category. The risk of malignancy and recommendations associated with each general category are not required but can be provided based on their own CNB-histology correlation or published studies.
I. Non-diagnostic or unsatisfactory
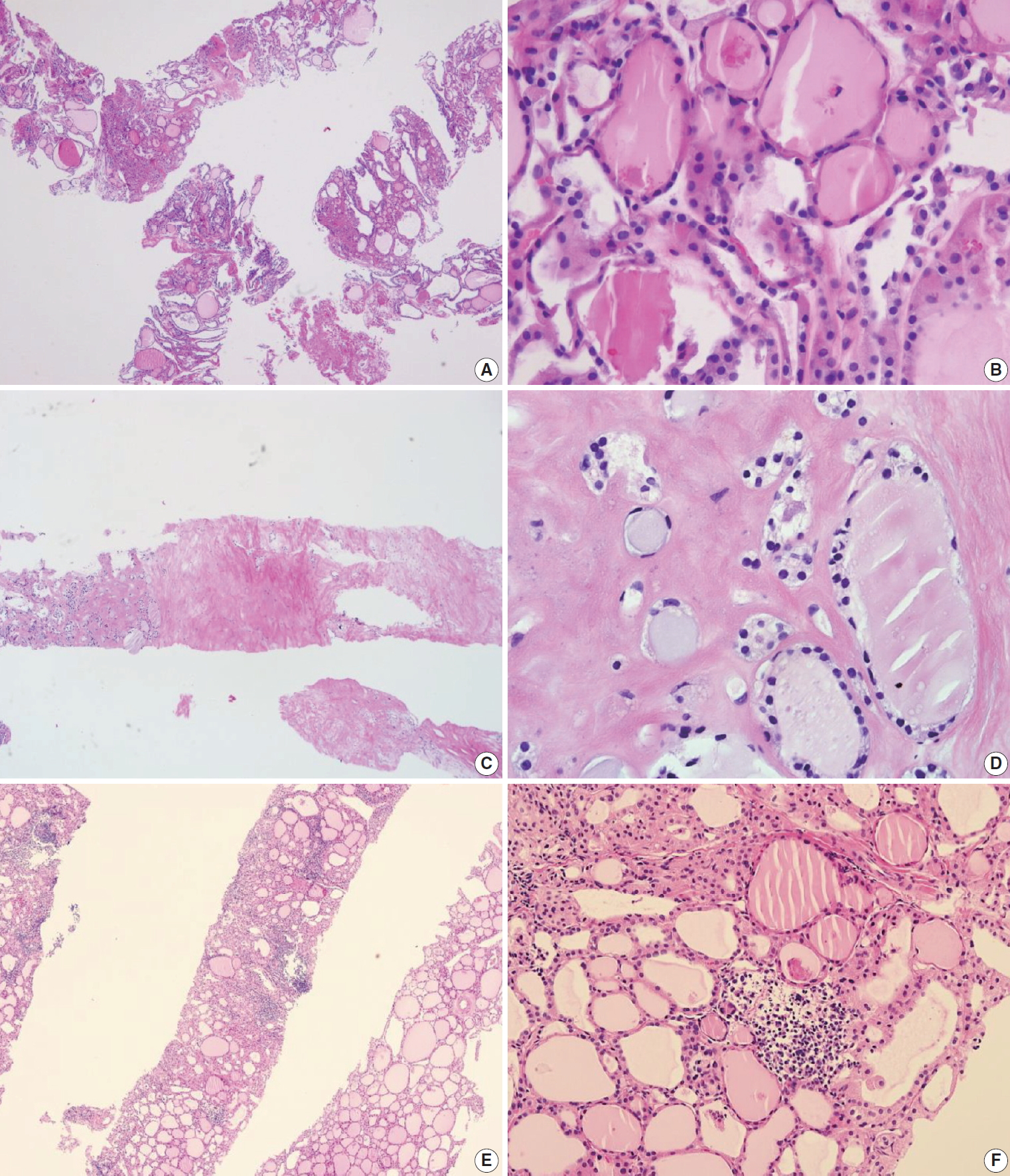
This category is used when the volume of biopsy specimen and the number of follicular cells is inadequate or unsatisfactory to obtain appropriate diagnosis or when the specimen is not representative of US imaging findings of the lesion. The adequacy of the specimen may depend on the inherent nature of the thyroid lesion as well as the experience and technique of the technician conducting the biopsy. This diagnosis is subjective as there is no consensus on the minimum number or amount of follicular components required for satisfactory biopsy specimen. Therefore, the report should explain why the sample is non-diagnostic or unsatisfactory. For example, the sample may be non-tumor tissue adjacent to thyroid (Fig. 2A), extrathyroid tissue alone (e.g., skeletal muscle and mature adipose tissues) as shown in Fig. 2B, an acellular specimen (Fig. 2C, D), or a blood clot. It may show other non-diagnostic or unsatisfactory findings. However, any CNB specimen containing atypical cells should not be considered non-diagnostic or unsatisfactory.

Examples of non-diagnostic specimens (category I) in thyroid core needle biopsy. The examples include non-tumor adjacent thyroid tissue only (A), extrathyroidal soft tissue only (B), and acellular sclerotic nodules (C, D).
Non-tumor adjacent thyroid tissue or extrathyroid tissue only implies that the thyroid lesion has not been sampled. For some benign follicular lesions with normofollicular structures (Fig. 2A), it may be difficult to assess specimen adequacy because follicular cells of the thyroid nodule show similar histological features with the adjacent thyroid tissue. It is important to determine the transition between the follicular lesion and the surrounding normal parenchyma and compare the pathologic findings with its US imaging results.
II. Benign
This category includes all benign thyroidal and nonthyroidal diseases. A CNB specimen can be classified, based on the specific lesion diagnosis, under the benign category. For example, the sample may be a benign follicular nodule, Hashimoto’s thyroiditis, subacute granulomatous thyroiditis, a nonthyroidal lesion (e.g., a parathyroid lesion, benign neurogenic tumors, benign lymph node), or other benign lesions.
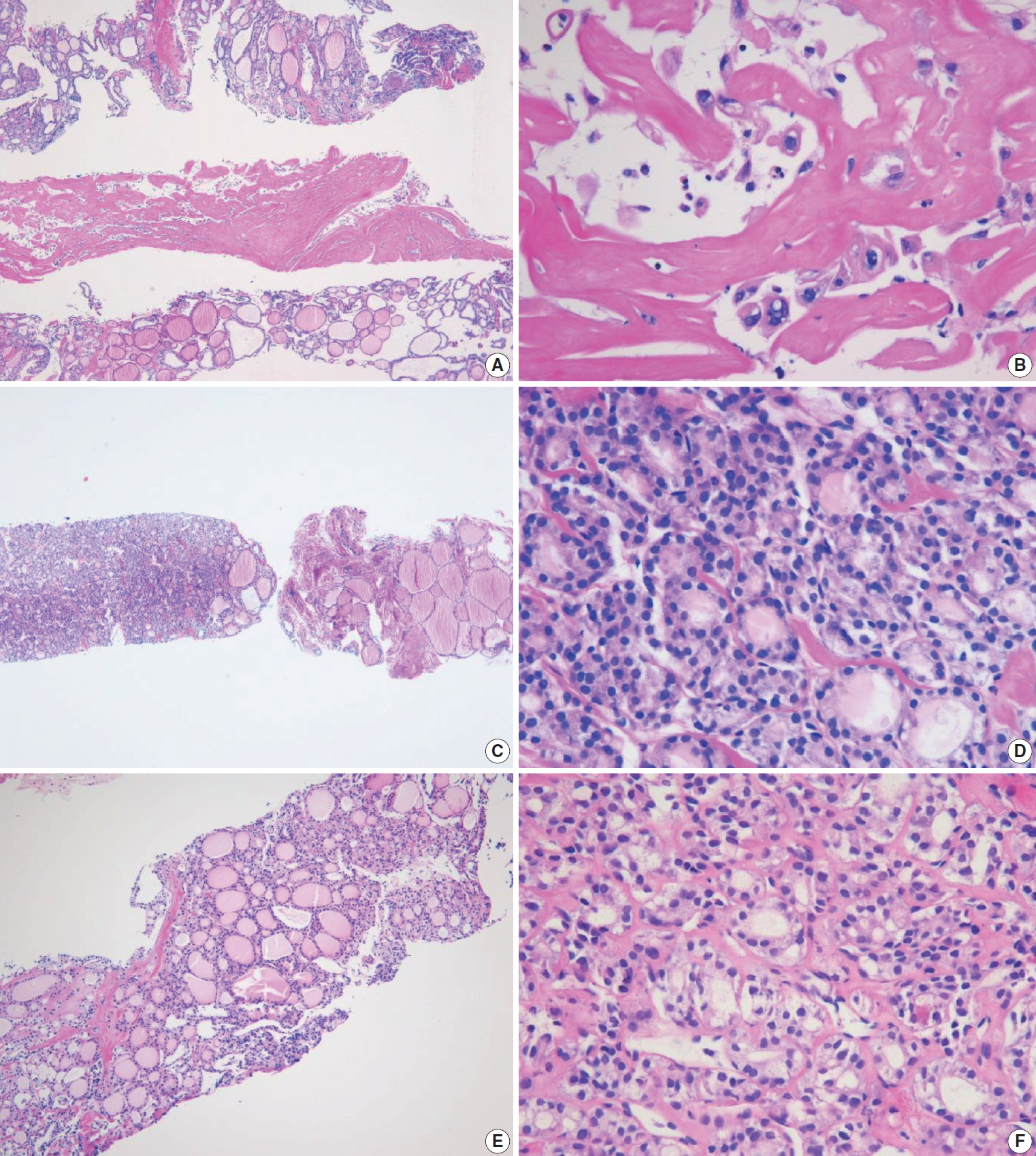
A benign follicular nodule encompasses nodular hyperplasia (adenomatoid nodule), colloid nodule, a nodule associated with Graves’ disease, nodular Hashimoto’s thyroiditis, and a subset of follicular adenoma. CNB specimens of these lesions show a benign-appearing normofollicular or macrofollicular structure and do not contain a well-defined fibrous capsule (Fig. 3A, B).
Examples of benign nodules (category II) in thyroid core needle biopsy. (A) The biopsy specimen shows follicular proliferative lesion without a distinct tumor capsule. (B) The high-power view of Fig. 3A shows benign-appearing follicular cells. This lesion can be interpreted as a benign follicular nodule. (C) The specimen is densely hyalinized and paucicellular. (D) The high-power view of Fig. 3C reveals benign-appearing follicular cells in the hyalinized stroma, suggesting a benign follicular nodule. (E) The specimen shows a Hürthle cell proliferative lesion. (F) The high-power view of Fig. 3E reveals Hürthle cell proliferation and lymphoid aggregates. This lesion should be interpreted as a benign Hürthle cell proliferative lesion rather than Hürthle cell neoplasm.
Most degenerating nodules are benign but they often show suspicious US findings and non-diagnostic or indeterminate cytologic results [69]. CNB of degenerating nodule according to the progression stage shows hemorrhagic materials in early phase, stromal fibrosis, and hyalinization infiltrated with chronic inflammatory cells after regression of hemorrhage in the middle phase, and paucicellular densely hyalinized stroma in late phase [69]. In a CNB specimen of degenerating nodule, the lesion can be diagnosed as a benign degenerating nodule if there are no atypical cells in the fibrous hyalinized and/or hemorrhagic background with scarce follicular cells (Fig. 3C, D) [69]. The atypia status can be determined by comparing the histologic features of follicular cells in both the central and peripheral regions included in the CNB specimen. Benign Hürthle cell lesions often occur in patients with Hashimoto’s thyroiditis and may resemble their neoplastic counterparts. The presence of lymphoid aggregates within the Hürthle cell lesion favors a diagnosis of benign (category II) over indeterminate Hürthle cell lesion (category IIId) or Hürthle cell neoplasm (category IVc) (Fig. 3E, F).
III. Indeterminate lesion
Cytological atypia and histological growth patterns in this category are of uncertain significance and insufficient to be classified under other diagnostic categories. The diagnostic category III “indeterminate lesion” corresponds to “AUS” or “FLUS” in TBSRTC. Most Korean pathologists prefer the term “indeterminate lesion” for category III in the histopathological diagnosis of CNB specimens while some pathologists prefer the term “AUS” [28,65]. Regardless of the terminology used, it is important for the pathologists to use only a single term, either indeterminate lesion, AUS, or FLUS.
The diagnosis of category III is appropriate when a follicular proliferative lesion shows focal nuclear atypia such as nuclear enlargement with pale chromatin, irregular nuclear membrane, and nuclear grooves in a background of predominantly benign-appearing follicles. A microfollicular proliferative lesion separated by a fibrous capsule from the surrounding normal parenchyma suggests a diagnosis of follicular neoplasm (see category IV). If a fibrous capsule or adjacent nonlesional tissue is not identified in a CNB specimen that shows a predominantly microfollicular or trabecular growth pattern, it is reasonable to classify the lesion under diagnostic category III because it is uncertain whether the nodule has a fibrous capsule. A multidisciplinary approach to a thyroid nodule can improve the preoperative diagnostic accuracy of FNA and CNB specimens [9]. Macrofollicular lesions are usually diagnosed as benign on FNA specimens. When US images show a dominant solitary nodule and the CNB specimen microscopically reveals a follicular proliferative lesion with a definite fibrous capsule, then the CNB specimen is diagnosed under category IV “follicular neoplasm,” even in case of a macrofollicular lesion.
Category III should be subclassified according to nuclear atypia, histologic growth patterns, cell type, and other histological features because the subclassification suggests different histological features and a risk of malignancy in the thyroid nodules [65]. A subcategory of nuclear atypia indicates that papillary thyroid carcinoma must be ruled out whereas architectural atypia cannot exclude follicular neoplasm. The following common scenarios may be encountered in case of follicular proliferative lesions.
IIIa. Indeterminate follicular lesion with nuclear atypia
Examples in this category include follicular proliferative lesions with focal nuclear atypia (Fig. 4A, B), follicular proliferative lesions with equivocal or questionable nuclear atypia, and atypical follicular cells embedded in a fibrotic or hyalinized stroma. This subcategory increases the risk of papillary carcinoma; however, the diagnosis does not reveal the characteristic histological features.
Examples of indeterminate follicular lesion (category III) in thyroid core needle biopsy. (A) The middle one in the three biopsy cores shows densely hyalinized stroma. (B) The high-power view of Fig. 4A reveals atypical cells with nuclear atypia embedded in the hyalinized stroma. This lesion can be interpreted as indeterminate follicular lesion with nuclear atypia (category IIIa). (C) The specimen shows microfollicular proliferation and adjacent thyroid parenchyma. The lesion does not contain a fibrous capsule that is required to establish a diagnosis of follicular neoplasm. (D) The high-power view of Fig. 4C reveals microfollicular growth and absence of nuclear atypia. This lesion can be interpreted as indeterminate follicular lesion with architectural atypia (category IIIb). (E) The specimen shows microfollicular proliferation lacking a fibrous capsule and adjacent thyroid parenchyma in the specimen. (F) The high-power view of Fig. 4E reveals microfollicular growth, thin fibrous bands, and mild nuclear atypia, suggesting indeterminate follicular lesion with nuclear and architectural atypia (category IIIc).
IIIb. Indeterminate follicular lesion with architectural atypia
Examples in this category include microfollicular proliferative lesions lacking a fibrous capsule or the adjacent nontumor tissue in the specimen; solid or trabecular follicular lesions lacking a fibrous capsule or adjacent nontumor tissue in the specimen (Fig. 4C, D). This subcategory exhibits no nuclear atypia. Some pathologists prefer category III “indeterminate follicular lesion” for a macrofollicular proliferative lesion with a definite fibrous capsule because a macrofollicular pattern is a characteristic of benign thyroid diseases.
IIIc. Indeterminate follicular lesion with nuclear and architectural atypia
In this category, follicular proliferative lesions show both nuclear and architectural atypia, but lack a fibrous capsule or the adjacent nontumor tissue in the specimen (Fig. 4E, F). Nuclear atypia is mild but lacking typical nuclear features of papillary carcinoma. Architectural atypia suggests microfollicular, solid, or trabecular proliferation. NIFTP is more frequently found in this subcategory [12].
IIId. Indeterminate follicular lesion with Hürthle cell changes
Follicular proliferative lesion is comprised exclusively of Hürthle cells, but the specimen lacks a fibrous capsule or the adjacent nontumor tissue (Fig. 5A, B). When Hürthle cell lesion is admixed with lymphoid aggregates, the CNB specimen should be diagnosed as benign Hürthle cell proliferative lesion (Fig. 3E, F).
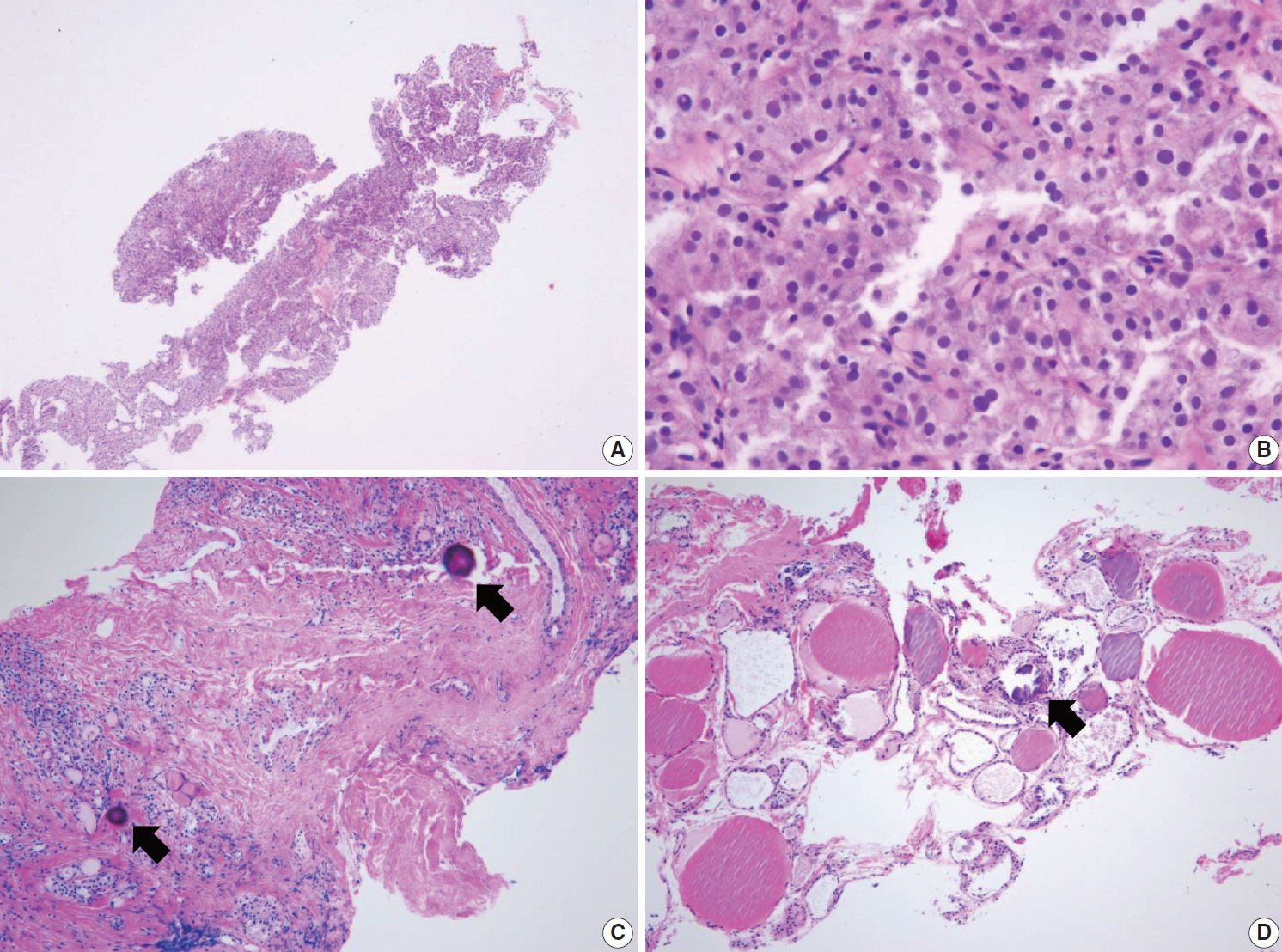
(A) Specimen showing Hürthle cell proliferative lesion lacking a fibrous capsule or adjacent nonlesional thyroid tissue. (B) The high-power view of Fig. 5A reveals trabecular growth of Hürthle cells without nuclear atypia, suggesting indeterminate follicular lesion with Hürthle cell changes (category IIId). Psammoma bodies (arrow) are found in a background of Hashimoto’s thyroiditis (C) and normal thyroid tissue (D). These specimens lack nuclear features of papillary carcinoma and can be interpreted as indeterminate lesion, not otherwise specified (category IIIe).
IIIe. Indeterminate lesion, not otherwise specified
This category includes follicular proliferative lesions with psammoma bodies but lacking nuclear features of papillary carcinoma; rare cases not explicitly described elsewhere in category III. Pseudo-psammomatous calcifications found in follicular lumens occur in Hürthle cell neoplasms and differ from true psammoma bodies found in papillary carcinoma (Fig. 5C, D).
IV. Follicular neoplasm
In CNB and FNA, the term “follicular neoplasm” is used to encompass neoplastic lesions with a follicular proliferative pattern (e.g., cellular nodular hyperplasia, follicular adenoma, NIFTP, follicular carcinoma, follicular variant of papillary carcinoma, follicular variant of medullary carcinoma) [26,86,87]. In TBSRTC, the terms “follicular neoplasm” and “suspicious for a follicular neoplasm” were equally acceptable but were not intended to separately denote two different interpretations. Only a single terminology is recommended for use in this category. The histological diagnosis of “follicular neoplasm” in a CNB specimen is based on the presence of a fibrous capsule and microscopic features that differ from the adjacent thyroid parenchyma (Fig. 6). In this category, follicular cells do not show typical nuclear features associated with papillary carcinoma. Papillary structure is often seen in hyperfunctioning follicular adenoma and follicular adenoma associated with papillary hyperplasia. The distinction is based on preserved cell polarity and lack of typical nuclear features of papillary carcinoma.
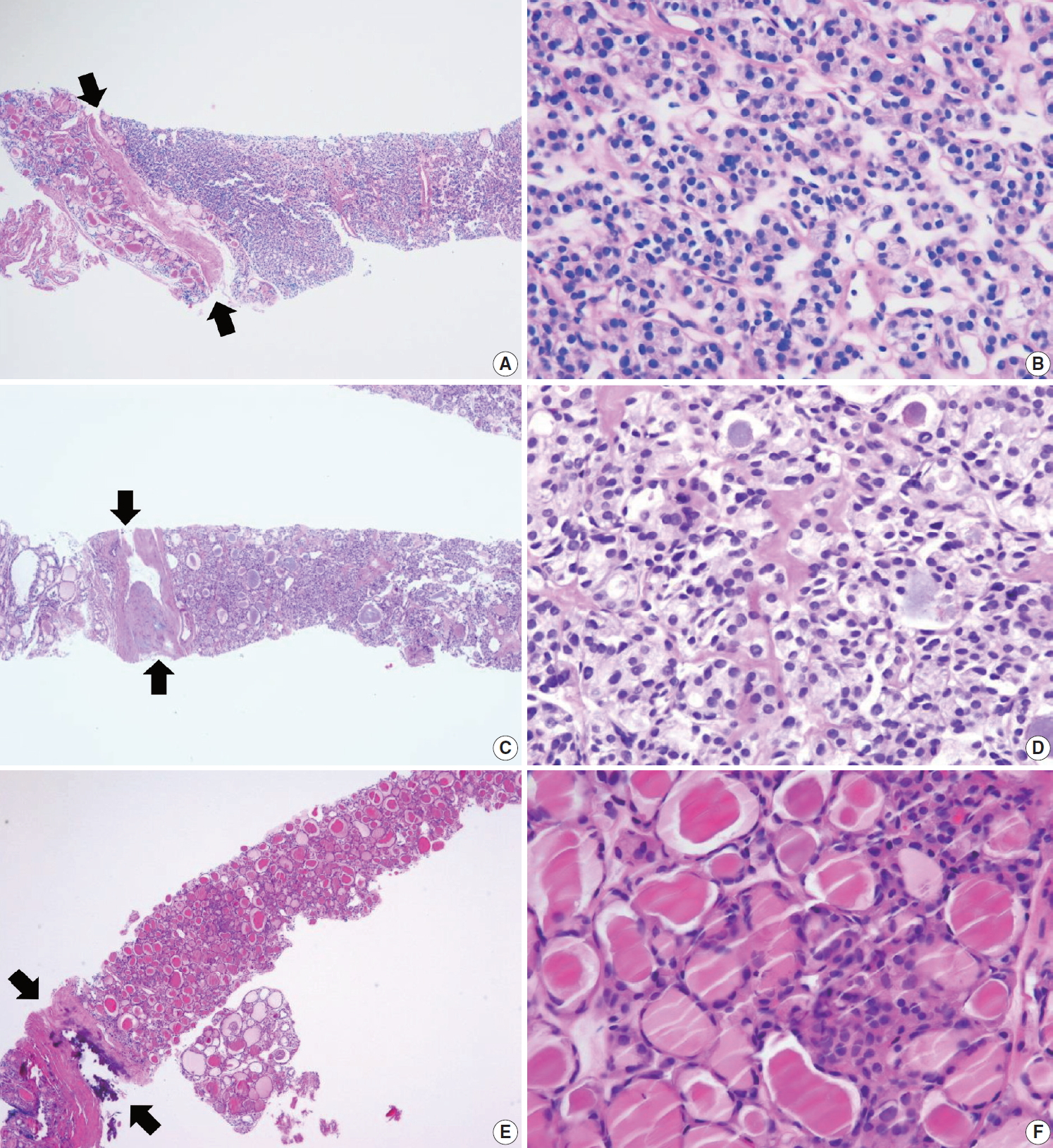
Examples of follicular neoplasm (category IV) in thyroid core needle biopsy. (A) The low-power view shows a follicular proliferative lesion with a fibrous capsule (arrows). (B) The high-power view of Fig. 6A reveals microfollicular growth pattern and no nuclear atypia. This lesion can be interpreted as follicular neoplasm, conventional type (category IVa). (C) The specimen shows a follicular proliferative lesion with a thick fibrous capsule (arrows). (D) The high-power view of Fig. 6B reveals microfollicular growth pattern and follicular cells with nuclear atypia, suggesting follicular neoplasm with nuclear atypia (category IVb). (E, F) Another example of follicular neoplasm with nuclear atypia (category IVb), in which, the lesion shows a fibrous capsule (arrows) nuclear atypia in focal areas (F).
It is important to identify a well-formed fibrous capsule for the diagnosis of follicular neoplasm in the CNB specimen. Fibrous capsule is composed of fibrous connective tissue, which separates tumor cells from the adjacent thyroid tissue. However, it may be difficult to distinguish histologically between fibrous tumor capsule and intralesional fibrous band in small CNB samples (Fig. 7). Correlation between US and pathological findings can be used to differentiate fibrous tumor capsule from intralesional fibrosis. The location of the specimen notch in the US images represents the contents of tissue sample.
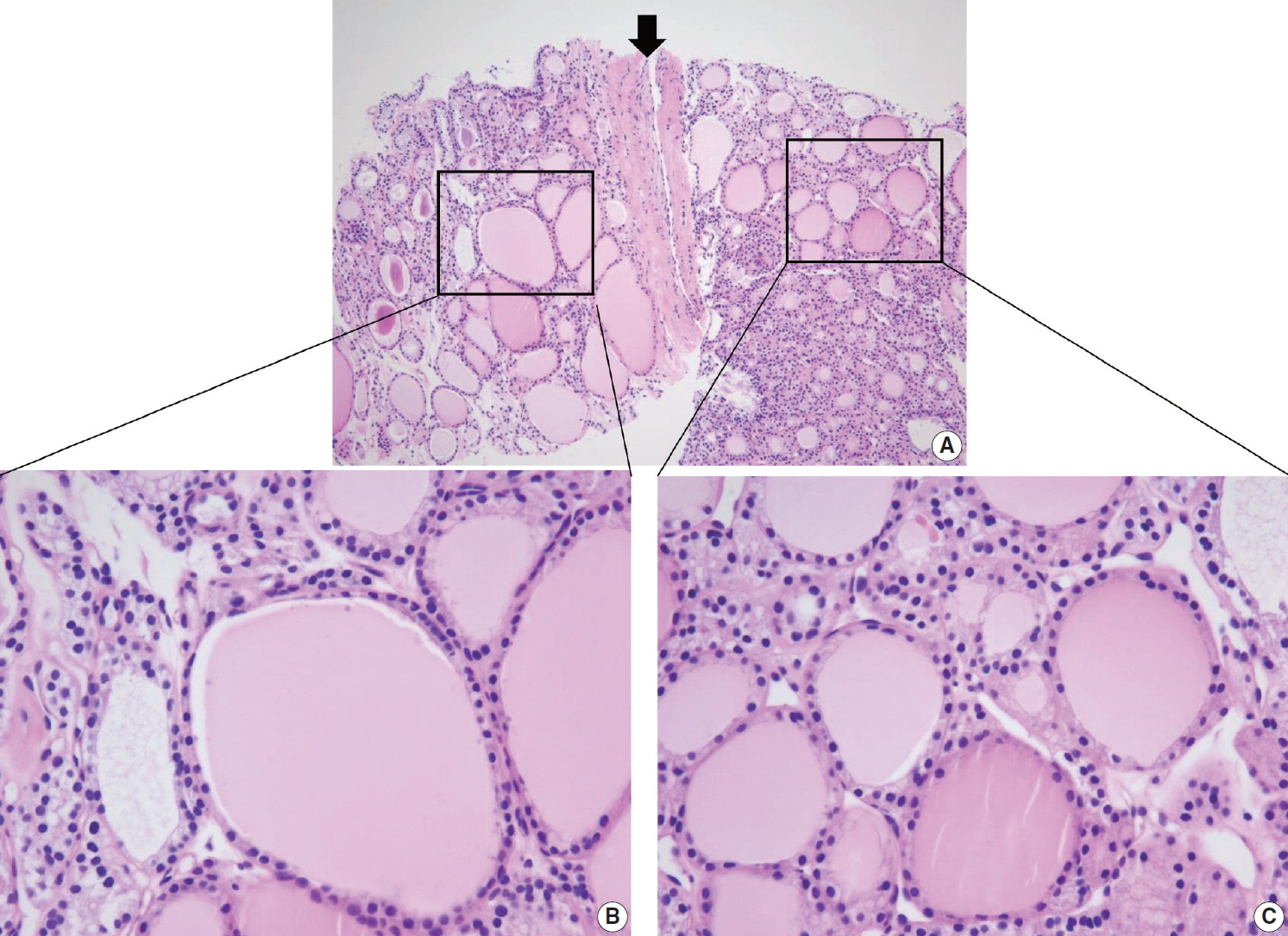
Differential diagnosis of benign follicular nodule with a fibrous capsule (arrow) and follicular neoplasm (A). The specimen is composed of follicular proliferative lesion (right side), fibrous capsule (arrow), and adjacent normal thyroid parenchyma (left side). The morphology of follicular cell population within the nodule (C) is identical to that of the adjacent thyroid parenchyma (B). The specimen should be interpreted as benign follicular nodule based on differences in growth pattern of the follicular neoplasm compared with the surrounding thyroid parenchyma.
The FNA diagnosis for follicular neoplasm is primarily based on the microfollicular or trabecular architecture and the lack of colloid. In a CNB specimen, the growth patterns of a follicular neoplasm can be microfollicular, normofollicular, solid, or trabecular when the tumor fibrous capsule is identified in the sample. A CNB cannot discriminate between a follicular carcinoma and a follicular adenoma because the diagnosis of these neoplasms requires examination of the entire tumor capsule. Neoplastic follicular and Hürthle cells show architectural and cytologic features that differ from the adjacent nontumor thyroid tissue (Figs. 6–8). Hürthle cell neoplasm is classified under a clinically and genetically distinct tumor category according to the 2017 WHO Classification of Tumors of Endocrine Organs [29]. Thyroid CNB is used as a screening test similar to thyroid FNA for the detection of neoplastic nodule that requires lobectomy for a definitive histological diagnosis of surgical specimens. It is recommended that the category IV should be subclassified into four subgroups according to nuclear atypia, histologic growth patterns, and cell type. The subclassification reflects differences in histology and the risk of malignancy in the thyroid nodules [88].
Thyroid core needle biopsy showing Hürthle cell neoplasm (category IVc). The images in the left (A, C) and right (B, D) columns show low-power findings and corresponding high-power views, respectively. The tumor capsule (arrows) can be thick (A) or thin (C).
IVa. Follicular neoplasm, conventional type
This category includes the following types of neoplasm: a microfollicular proliferative lesion with a fibrous capsule (Fig. 6A, B); a mixed microfollicular and normofollicular proliferative lesion with a fibrous capsule; a solid or trabecular follicular lesion with a fibrous capsule; a follicular proliferative lesion showing papillary structure and fibrous capsule but lacking nuclear features of papillary carcinoma.
IVb. Follicular neoplasm with nuclear atypia
Focal nuclear atypia in follicular neoplasm raises the possibility of NIFTP and follicular variants of papillary carcinoma (Fig. 6C, D) [57,88-90]. Before the NIFPT era, this subcategory frequently turned out to be a follicular variant of papillary carcinoma after surgery [89,90].
IVc. Hürthle cell neoplasm
A Hürthle cell proliferative lesion with a fibrous capsule should be diagnosed in this category (Fig. 8). Hürthle cells exhibiting characteristic nuclear features of papillary carcinoma are papillary carcinoma cells, and therefore should not be diagnosed as Hürthle cell neoplasms. Hashimoto’s thyroiditis often presents as a solitary nodule or as a dominant thyroid nodule. CNB sampling from a nodule of Hashimoto thyroiditis shows Hürthle cell proliferation in microfollicles, macrofollicles, or trabecula, which may contain a fibrous capsule surrounding the lesion that could be misinterpreted as Hürthle cell neoplasms (Fig. 3E, F). Careful search for lymphocyte and plasma cell infiltration is essential to distinguish such cases from Hürthle cell neoplasm. The Hürthle cell proliferative lesion admixed with lymphocytes and plasma cells should be diagnosed as benign Hürthle cell proliferative lesion rather than Hürthle cell neoplasm or indeterminate follicular lesion with Hürthle cell changes.
IVd. Follicular neoplasm, not otherwise specified
This category includes rare cases not explicitly described elsewhere in category IV.
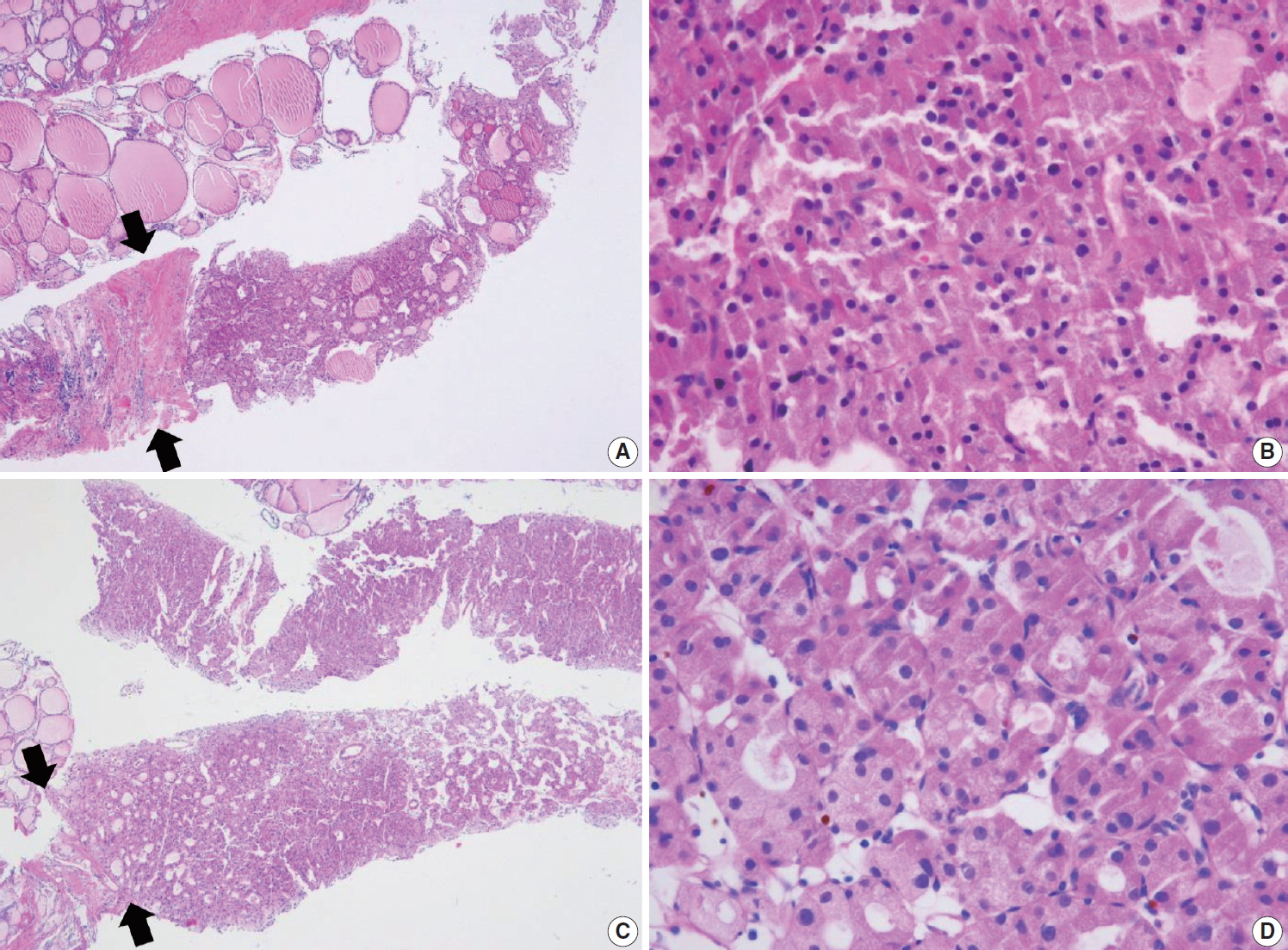
V. Suspicious for malignancy
The diagnosis of “suspicious for malignancy” is established when histological features are strongly suspicious for malignancy but are insufficient or equivocal for a definitive diagnosis of malignancy. In this category, a lesion may be suspicious for papillary thyroid carcinoma (Fig. 9), medullary thyroid carcinoma, poorly differentiated thyroid carcinoma, metastatic carcinoma, lymphoma, or show other suspicious findings. The diagnostic uncertainty in this category is generally caused by suboptimal sampling or low cellularity.
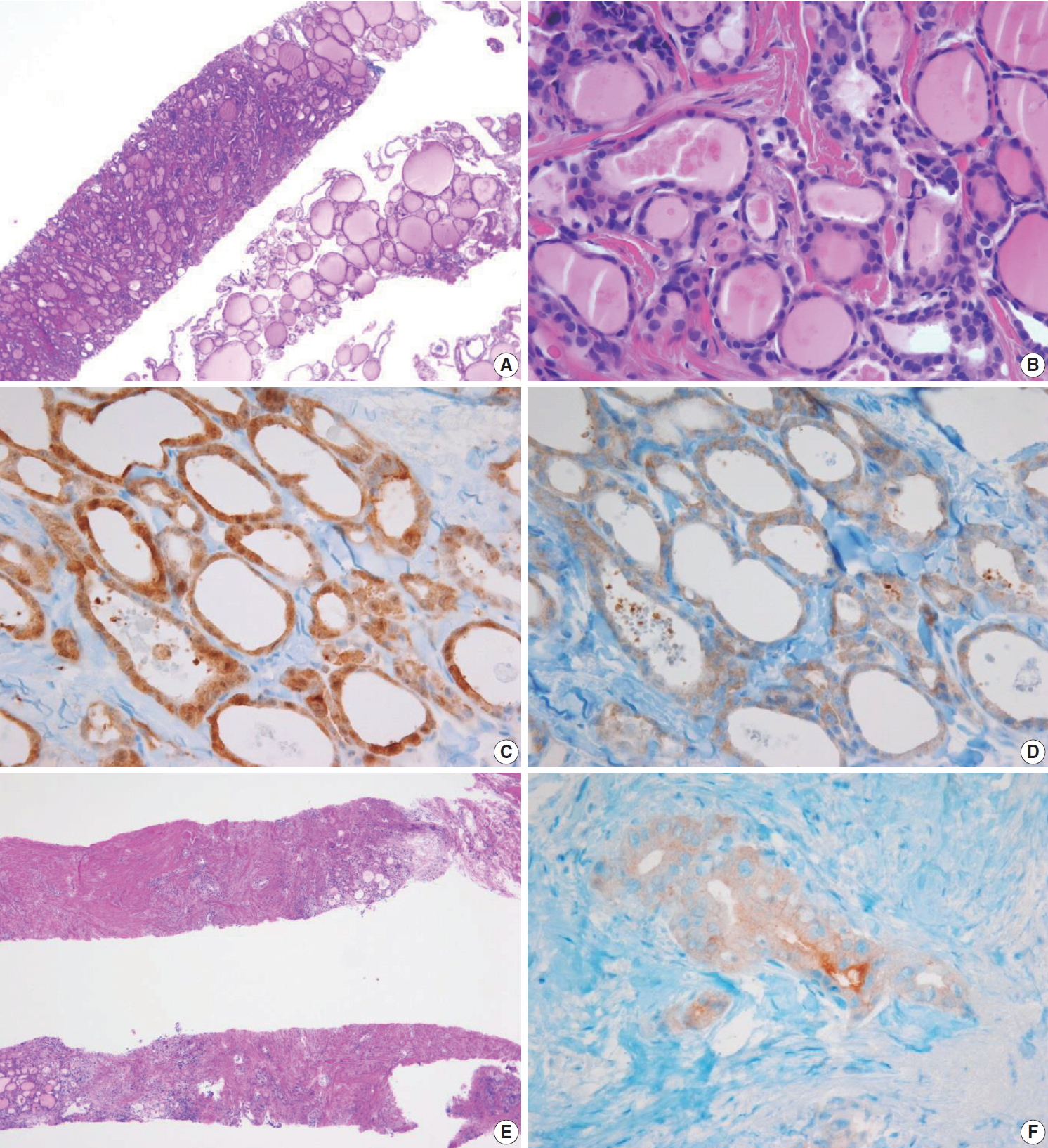
(A) The specimen shows a follicular proliferative lesion with an ill-defined border showing a different morphology compared with adjacent normal thyroid tissue. (B) The high-power view in Fig. 9A reveals atypical follicular cells with nuclear atypia and fibrous bands. These morphological features can be interpreted as suspicious for papillary carcinoma (category V). In this case, immunohistochemical stains for galectin-3 (C) and BRAF VE1 (D, F) facilitate the diagnosis of papillary carcinoma. (E) A paucicellular, hyalinized follicular lesion exhibits morphological features pathognomonic for papillary carcinoma. The diagnosis of the lesion can be established as papillary carcinoma when atypical follicular cells in the specimen are positive for BRAF VE1 immunostaining (F).
Follicular variants of papillary carcinoma and NIFTP share histologic features of follicular growth and nuclear features of papillary carcinoma, and cannot be discriminated by FNA alone. The diagnosis of papillary carcinoma by FNA requires either intranuclear pseudoinclusions, papillae, or psammoma bodies. In a CNB specimen, both areas of tumor and nontumor can be histologically evaluated. When a follicular proliferative lesion with nuclear features of papillary carcinoma shows capsular invasion in CNB specimens, the findings should be interpreted as papillary carcinoma. In the absence of capsular invasion, it should be interpreted as follicular neoplasm with nuclear atypia. However, when a fibrous capsule or adjacent nonlesional tissue is not identified in the CNB specimen that shows a predominantly follicular growth pattern and nuclear features of papillary carcinoma, the findings are best interpreted as suspicious for papillary carcinoma.
Ancillary immunohistochemical or molecular studies facilitate the diagnosis of CNB specimens with suspected malignancy. An immunostaining panel consisting of galectin-3, HBME1, cytokeratin 19, or CD56 facilitates the diagnosis of lesions suspicious for papillary thyroid carcinoma (Fig. 9) [91-93]. A combination of at least two immunostaining markers is recommended to improve the diagnostic accuracy [92]. The BRAF V600E mutations detected via molecular test or immunohistochemistry for BRAF VE1 antibody strongly suggest a diagnosis of papillary carcinoma (Fig. 9) [91]. A diagnosis of medullary thyroid carcinoma can be confirmed with positive immunostaining for calcitonin on the CNB specimen. A diagnosis of lymphoma is established using immunophenotyping studies on a CNB specimen that is suspicious for lymphoma [56].
VI. Malignant
Most thyroid malignancies, except for follicular carcinoma/Hürthle cell carcinoma, exhibit typical histological features and are easily diagnosed as a malignancy on a CNB specimen. The category of thyroid malignancies includes the following diagnoses: papillary thyroid carcinoma (Fig. 10A, B), poorly differentiated carcinoma (Fig. 10C, D), medullary thyroid carcinoma (Fig. 10E, F), anaplastic thyroid carcinoma (Fig. 11A–C), lymphoma (Fig. 11D–F), and metastatic carcinoma.

Core needle biopsies of malignant thyroid tumors (category VI). (A, B) Diffuse sclerosing variant of papillary carcinoma. (C, D) Poorly differentiated carcinoma shows solid, trabecular, and insular growth patterns and mitosis (arrows) under high-power field. Medullary carcinoma shows typical histologic features under low-power field (E) and high-power field (F). Nuclei of tumor cells are round to oval and carry coarsely granular chromatin. The cytoplasm is finely granular eosinophilic to amphophilic.
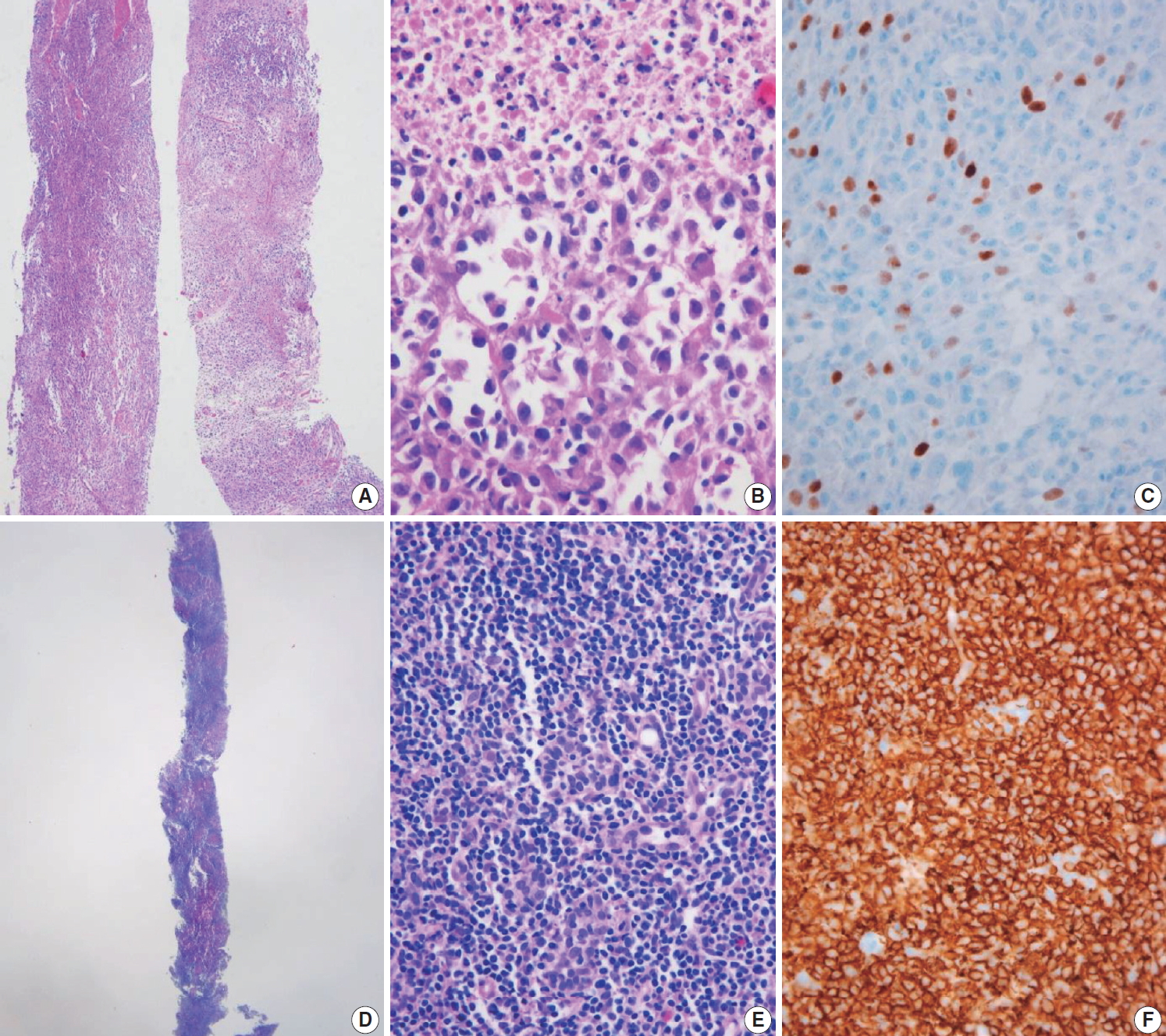
Core needle biopsies of malignant thyroid tumors (category VI). (A) Anaplastic thyroid carcinoma shows diffuse growth infiltration, with no papillary or follicular structure. (B) The tumor cells show marked nuclear pleomorphism and necrosis. (C) PAX8 immunostaining is focally positive in this anaplastic thyroid carcinoma. (D) In extranodal marginal zone lymphoma of mucosa-associated tissue, the lymphoma cells infiltrate follicles and destroy normal parenchyma. (E) Lymphoepithelial lesions are shown. (F) Tumor cells are diffusely positive for CD20.
COMMON PITFALLS IN THE INTERPRETATION OF CORE NEEDLE BIOPSY
The follicular cells in a CNB specimen appear smaller and show darker chromatin than is typical in a surgical specimen (Fig. 12A, B). Therefore, the nuclear features of papillary carcinoma are less prominent in a CNB specimen (Fig. 12C, D).
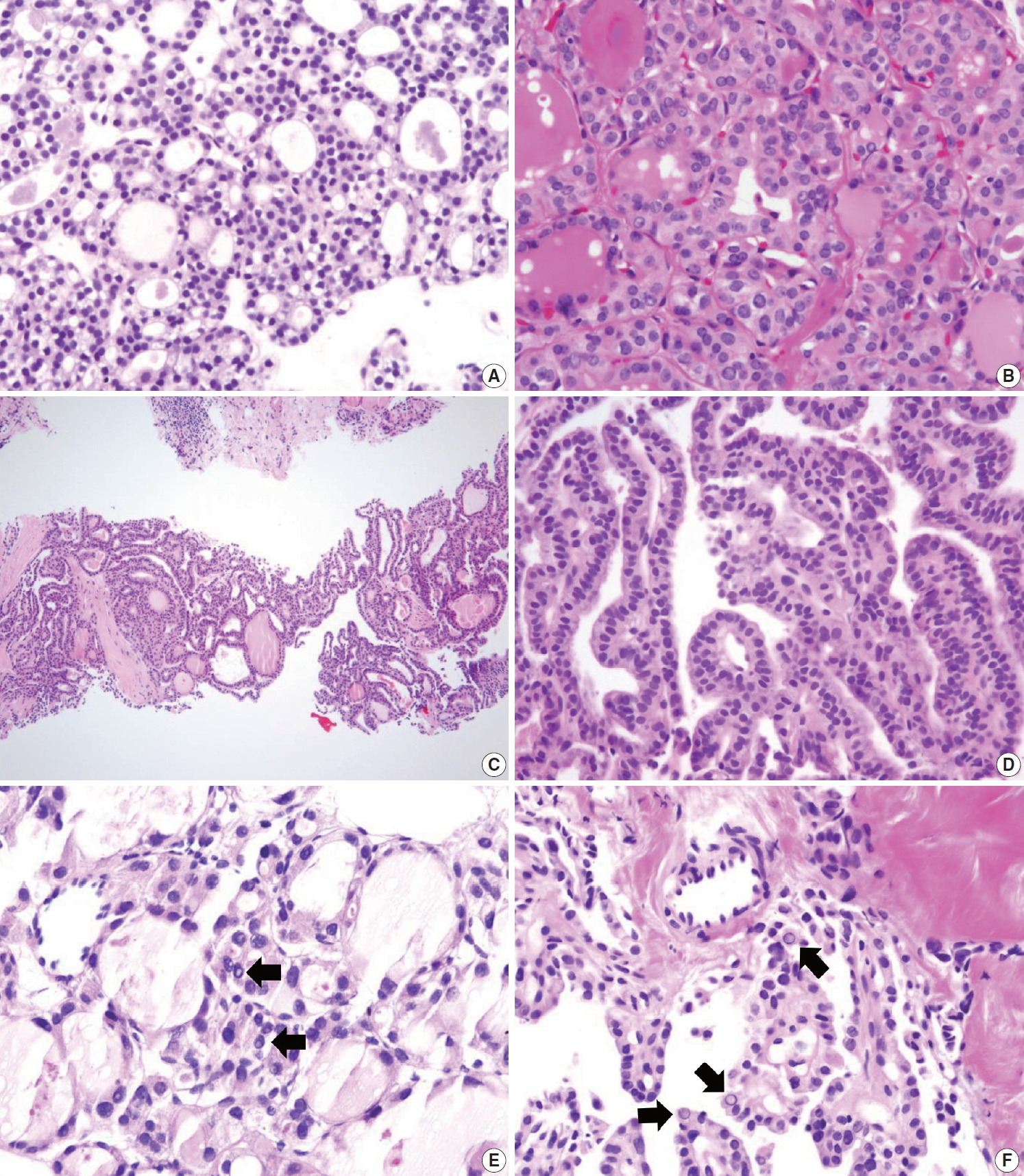
Diagnostic pitfalls in thyroid core needle biopsy. Follicular cells are smaller and darker in core needle biopsy (A) compared with the resected specimen (B) in the same thyroid nodule. (C) The core needle biopsy shows histologic features of papillary carcinoma. (D) The high-power view of Fig. 12C shows nuclear overlapping and crowding. Nuclear chromatin is finely dispersed but nuclear clearance is not as prominent as in papillary carcinoma derived from routine formalin-fixed sections of the resected specimen. (E) Nuclear pseudoinclusion-like nuclear bubbles or vacuoles (arrows) are often seen in the core needle biopsy specimen of benign follicular nodule. These structures show empty appearance and lack a thick nuclear membrane rim. (F) Core needle biopsy of papillary carcinoma shows intranuclear pseudoinclusions (arrows) exhibiting the characteristic cytoplasmic staining and are sharply demarcated by a nuclear membrane.
Nuclear artifacts that mimic intranuclear cytoplasmic pseudoinclusions in papillary carcinoma may also be present in benign follicular cells (Fig. 12E). The artifactual vacuoles or bubbles are irregular in shape and appear pale on staining, whereas intranuclear pseudoinclusions in papillary carcinoma are round and sharply delineated by the rim of the nuclear membrane, and exhibit cytoplasmic staining quality (Fig. 12F).
CONCLUSION
US-guided CNB represents an alternative procedure to overcome the challenges associated with FNA for the diagnosis of thyroid nodules. The main goal of thyroid CNB is to obtain a large amount of thyroid lesion with minimal morbidity, and to triage patients with thyroid disease who need surgical management. These practical guidelines serve as a clinical guide for successful thyroid CNB and provide a standardized system for pathology reporting of CNB specimens.
Notes
Author contributions
Conceptualization: CKJ, JHB.
Data curation: CKJ, JHB, DGN.
Formal analysis: CKJ, JHB, DGN.
Funding acquisition: CKJ.
Investigation: CKJ, JHB, DGN, YLO, HCK.
Methodology: CKJ, JHB, DGN, KHY, YLO, HCK.
Project administration: CKJ.
Resources: CKJ, JHB, DGN, KHY, YLO, HCK.
Supervision: CKJ, JHB.
Validation: CKJ, JHB, DGN, KHY, YLO, HCK.
Visualization: CKJ, JHB, DGN, KHY, YLO, HCK.
Writing—original draft: CKJ, JHB, DGN.
Writing—review & editing: CKJ, JHB, DGN, KHY, YLO, HCK.
Conflicts of Interest
C.K.J. is the editor-in-chief of Journal of Pathology and Translational Medicine. He serves on the Board of Directors of the KTA and the Korean Society of Pathologists. J.H.B. is the president of the KSThR. D.G.N. serves on the KTA and KSThR Board of Directors. K.H.Y. served as a Director General of the KTA Board of Directors during 2017–2019. H.C.K. serves as a Director of Clinical Practice Guidelines Development Committee of the Korean Thyroid Association.
Funding
These practical guidelines were supported by KTA without support from any commercial sources. This project was also partially supported by a grant (2017R1D1A1B03029597) from the Basic Science Research Program through the National Research Foundation of Korea.
Acknowledgements
The authors would like to thank the members of KTA, KSThR, and the Endocrine Pathology Study Group of the Korean Society of Pathologists for their valuable expertise and suggestions throughout this study. This document was approved by the Clinical Practice Guidelines Development Committee of the KTA in October 2019. The following professional organizations reviewed and endorsed the final document: KSThR, Korean Endocrine Pathology Study Group, and Korean Endocrine Society.