Aneurysmal bone cyst: a review
Article information
Abstract
Aneurysmal bone cyst (ABC) is a benign locally destructive bone neoplasm composed of multi-loculated blood-filled cystic spaces. The most common sites of involvement are the meta-diaphysis of the long bones and posterior elements of the vertebrae. Secondary, ABC-like changes can complicate a variety of other benign and malignant primary bone neoplasms, including giant cell tumor, fibrous dysplasia, and osteosarcoma. About two-third of primary ABCs have a rearrangement of the USP6 gene, which is not present in the ABC-like changes that occur secondary to other primary bone tumors (i.e., secondary ABC). Primary ABC of bone carries a variable but generally high rate of local recurrence. This paper provides an overview of the pathophysiology, clinical presentation, radiographic and pathologic findings, treatment, and prognosis of ABC.
Aneurysmal bone cyst (ABC) is a benign blood-filled cystic neoplasm of bone with a broad spectrum of skeletal involvement. It can present as a primary tumor, but ABC-like changes can also complicate other neoplastic diseases of bone.
ABC is a rare neoplasm with an annual prevalence of 0.32 per 100,000 young population [1], 0.14 per 100,000 general population [2], and comprising about 2.5% of all bone tumors [3]. It has an equal distribution among male and female patients [4,5], and most commonly is seen in skeletally immature patients especially in the first two decades of life [2].
Historically, it was thought that an ABC develops as the result of an underlying vascular event; increased venous blood flow; or a reaction to prior trauma [6]. However, in light of the recent molecular findings of the recurrent rearrangement involving the USP6 gene (chr.17p13.2 locus) [7-9]. ABC is now considered a neoplasm rather than a reactive lesion.
Although ABC can affect any bone in the body, the craniofacial bones, vertebrae (particularly the posterior elements) and metaphysis of long tubular bones in the upper and lower extremities are more commonly involved [3]. Other less common sites of involvement include small tubular bones of hands and feet, tarsal bones, scapula, and pelvic bones.
Patients usually present with pain and swelling of variable duration at the site of involvement. Rarely, the initial presentation is pathologic fracture specifically in the major long tubular bones of the extremities [10]. In the vertebral lesions, symptoms of compression of the spinal cord or nerve roots may be the initial presentation [11,12].
The diagnosis of ABC requires the correlation of clinical, radiographic, and histologic findings and to distinguish the primary from a secondary form of the disease.
IMAGING
The radiographic features of ABC are quite distinct and aid in diagnosing the disease. Conventional radiographs show an eccentric radiolucent lesion with expansile remodeling of bone. A thin surrounding rim of the periosteum and sub periosteal bone is usually present. The cyst wall trabeculae impart the multilocular appearance (Fig. 1). In the vertebral column, ABC most commonly involves the posterior neural arch and can produce an eccentric “blowout” lesion [3]. In small tubular bones of hands and feet, the characteristic “finger-in-the-balloon” sign might be present.
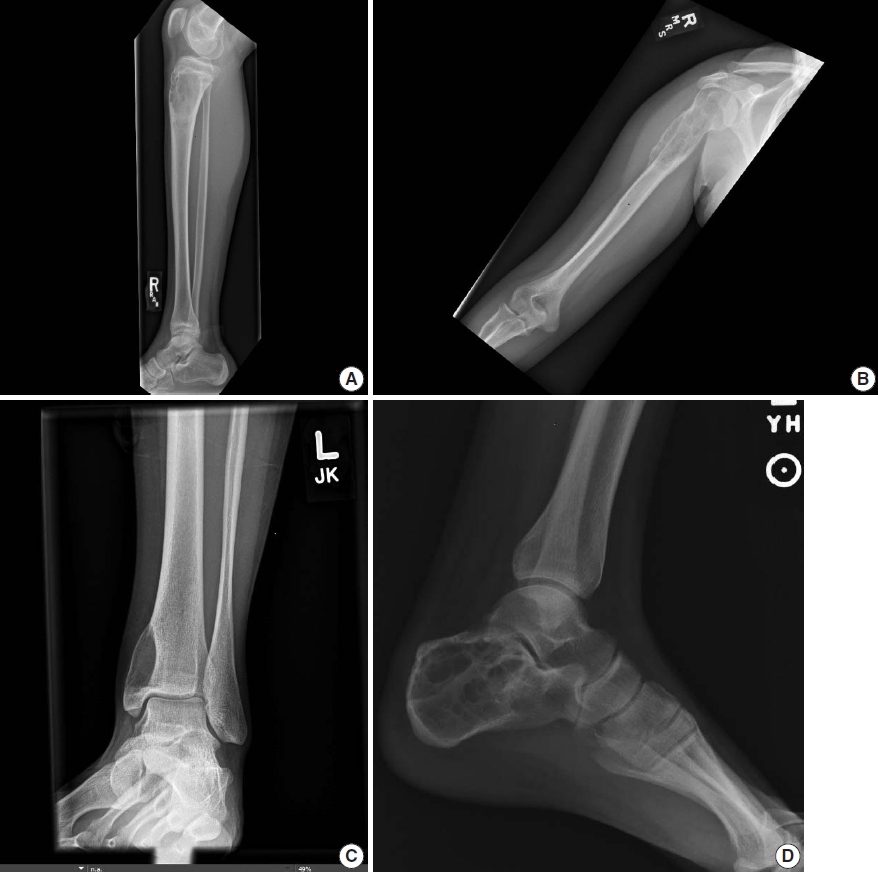
Plain radiography of aneurysmal bone cyst. (A) X-ray demonstrates a lytic lesion in the proximal metaphysis of the tibia with slight expansile features and lucency extending through the cortex. (B) X-ray shows an expansile lucent lesion centered in the medullary cavity of the proximal humeral metaphysis with cortical thinning. (C) X-ray shows an eccentric lucent lesion of the medial side of the distal tibia. (D) X-ray shows an expansile multiloculated lucent lesion of the calcaneus.
Computed tomography shows a well-delineated lytic lesion, usually with a thin surrounding rim of reactive bone (Fig. 2A). Occasionally, fluid-fluid levels are visible. However, the best imaging modality to identify the fluid-fluid levels is magnetic resonance imaging. The cysts usually demonstrate variable signal intensity with a rim of low T1 and T2 signal. T1 post-contrast sequence may show some enhancement of septations (Fig. 2B–D) [13,14].
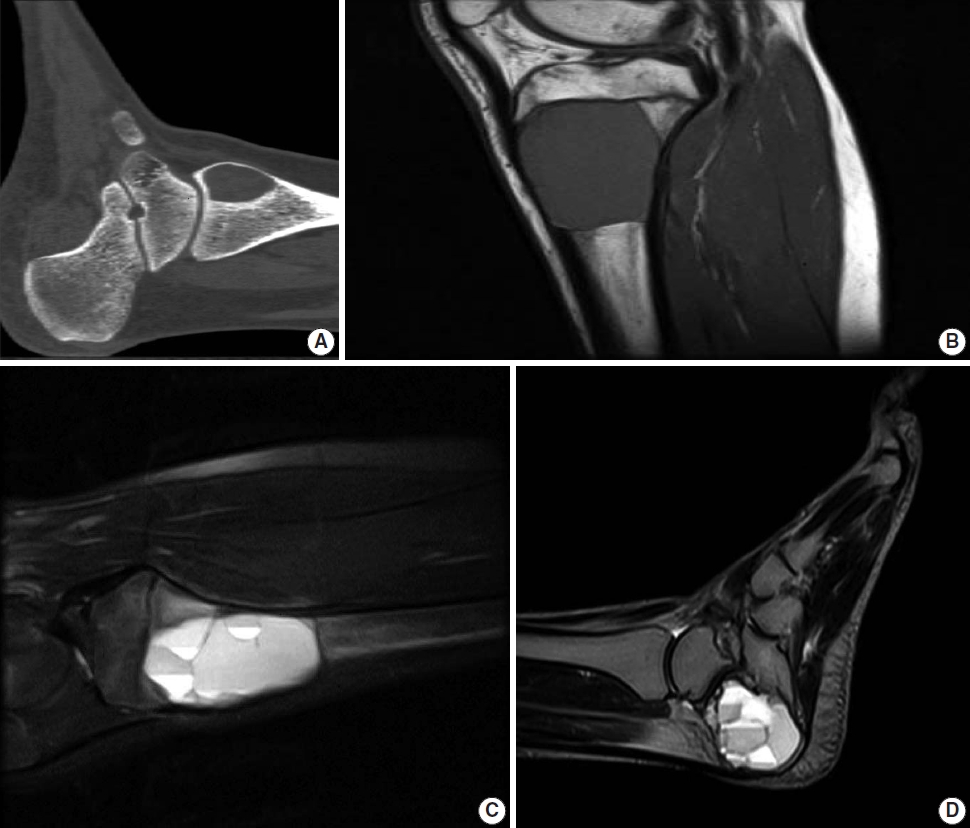
Computed tomography (CT) and magnetic resonance imaging (MRI) of aneurysmal bone cyst. (A) CT scan shows a well-demarcated ovoid area of lucency within the medial aspect of the distal tibia. (B, C) MRI shows an expansile well-demarcated cystic lesion of the proximal tibia with fluid-fluid levels. (D) Sagittal view T2 MRI demonstrates multiple fluid-fluid levels of the cyst involving the calcaneus bone.
Isotope scan shows a peripheral uptake with central photopenia, which imparts a “donut sign” appearance. This appearance is not specific for ABC and can also be seen in other bone lesions with ABC-like changes such as chondroblastoma and giant cell tumor of bone.
PATHOLOGY
Grossly, an ABC appears as a well-demarcated spongy hemorrhagic lesion with variably-sized multiloculations. The cystic spaces are variable in size ranging from less than one millimeter to several centimeters [3]. It has irregular, sharply demarcated borders with a thin shell of reactive bone around it. A variable amount of solid component might be present, particularly in the digits (Fig. 3).
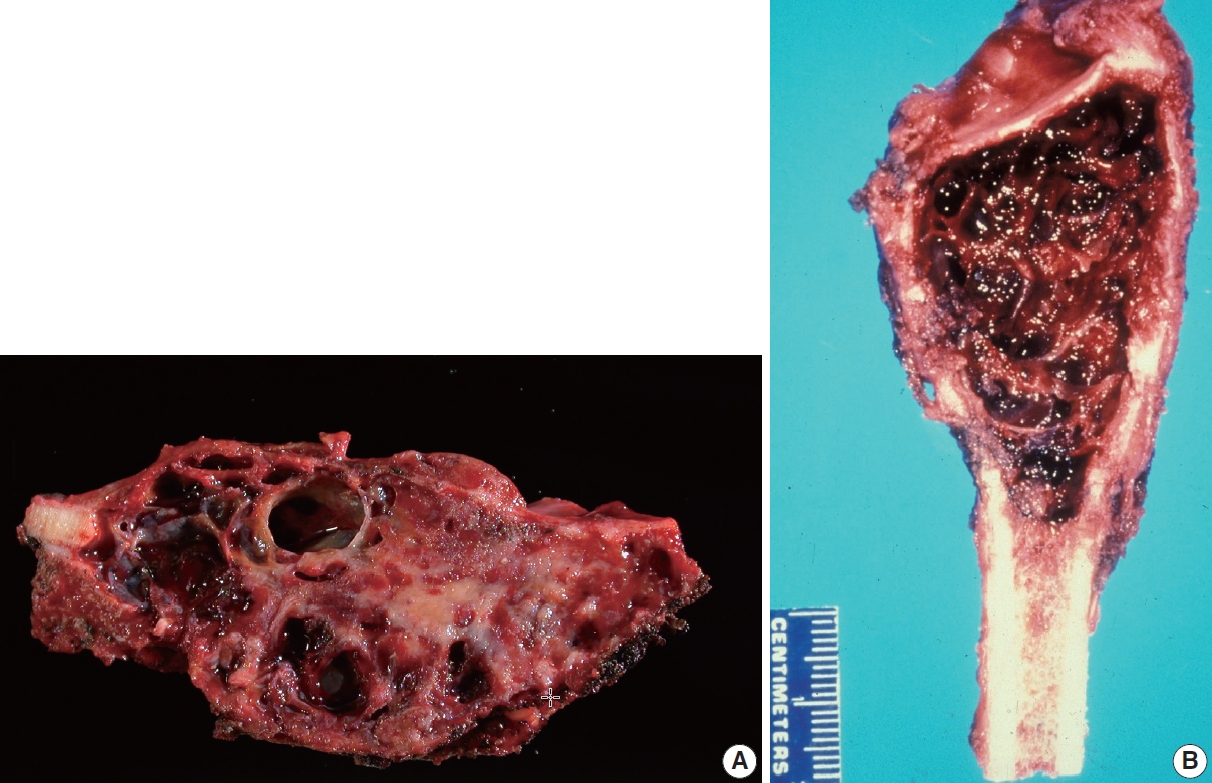
Gross findings of aneurysmal bone cyst (ABC). (A) ABC involving the distal fibula, showing an expansile lesion with multiple bloodfilled cystic spaces. (B) ABC involving tibia, showing an expansile lesion with multiple blood-filled cystic spaces.
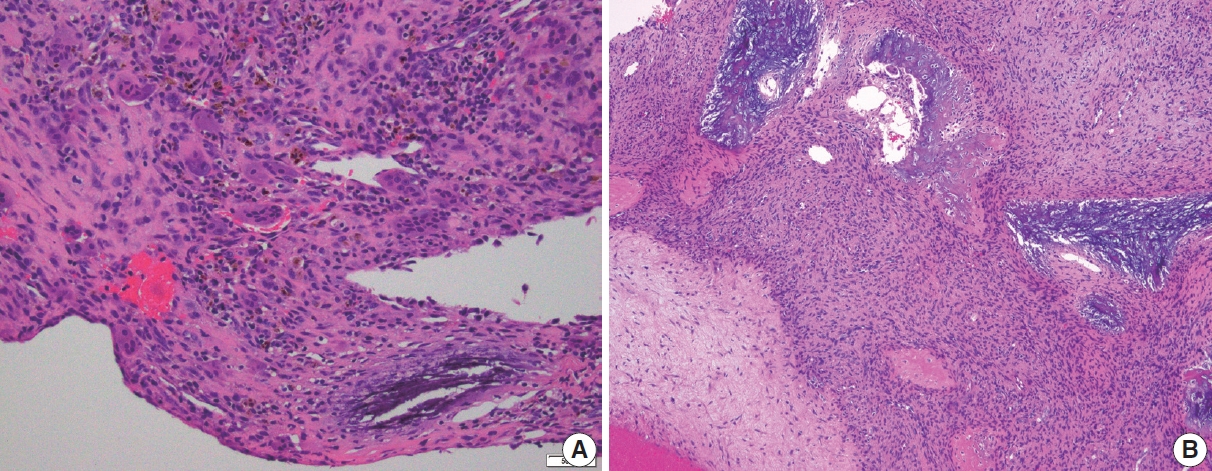
When encountered in an intraoperative consultation (frozen section), ABC is characterized by small fragments of cellular septa containing fibroblast-like stromal cells, osteoclast-like giant cells, and reactive woven bone that frequently displays osteoblastic rimming. While mitoses are typically easily identified, atypical mitoses and cytologic atypia are not present, and when these features are encountered on a frozen section they warrant a detailed discussion with the surgeon (Fig. 4).
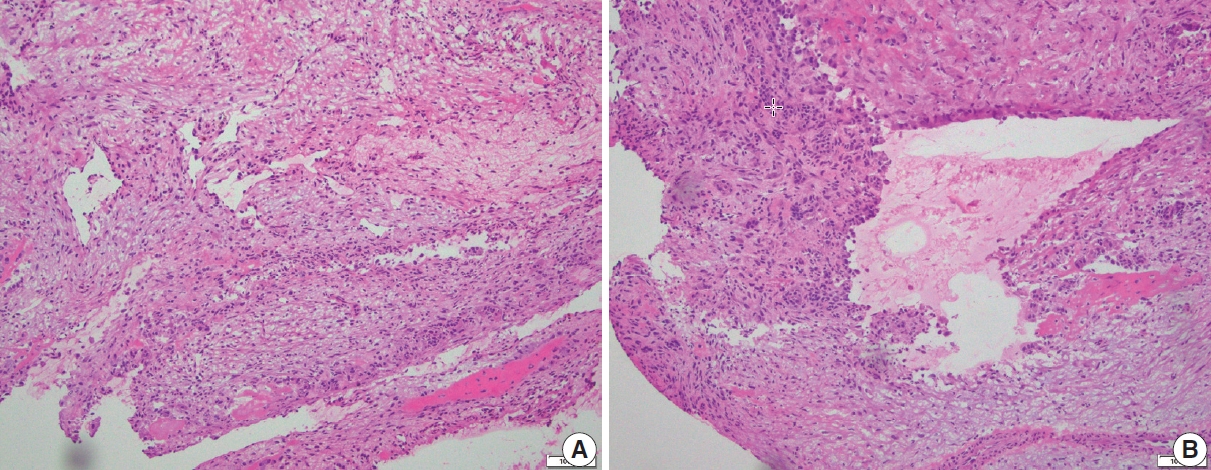
Frozen section findings of aneurysmal bone cyst. (A) Frozen section of a multiloculated cystic lesion of the distal tibia. Cystic space with flat attenuated lining and increased stromal giant cells. (B) The solid area shows scattered giant cells in the background of fibrovascular stroma. Cellular atypia is not present.
ABC is almost always received as curetted material rather than an en-bloc resection. As a rule, the curetted material must be entirely submitted for histologic evaluation. Low-power microscopic examination shows a multiloculated cystic lesion with collapsed cyst walls within the background of blood and hemorrhage. The septa show mixed inflammatory cells, reactive fibroblasts, woven bone, and some vasoformative foci; however, the cyst walls lack a true lining. Characteristic calcified basophilic material, referred to as “blue reticulated chondroid-like material” might be present within the cyst walls [15]. The cysts are generally devoid of any lining but some flattened endothelial-like cells can be present. Osteoclast-type giant cells are found in clusters with increased numbers within the cyst wall. Mitoses can be easily identified, but atypical mitoses are not. Necrosis and cytologic atypia are not the features of ABC (Figs. 5, 6). There is no specific immunohistochemical stain for the diagnosis of ABC.
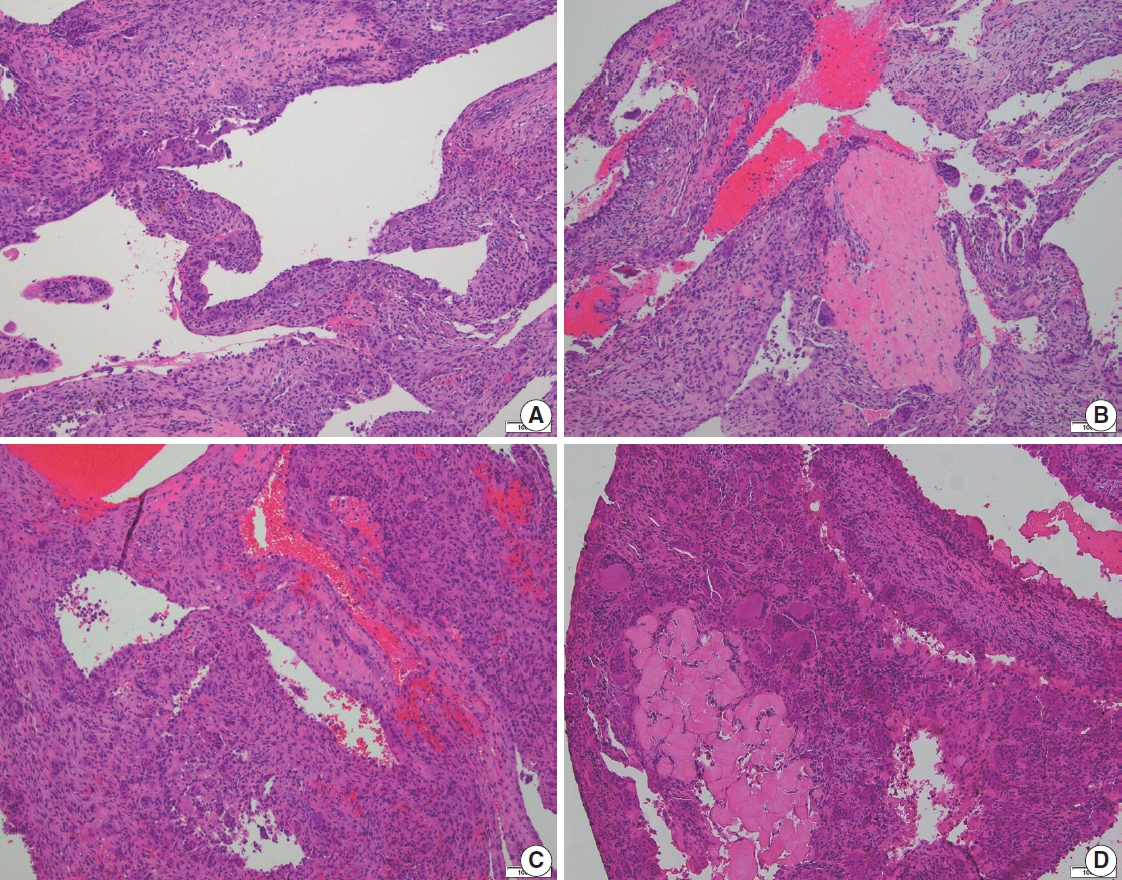
Histologic features of aneurysmal bone cyst. (A) Irregular cystic spaces with multi-nucleated giant cells are present. (B) Multiple irregular cystic spaces, some filled with blood, and a variable number of giant cells in the cyst wall. (C) Blood-filled cystic spaces with cellular giant cell-rich cyst wall. (D) Cystic spaces and portion of cyst wall with scattered giant cells. No significant atypia is present.
MOLECULAR AND CYTOGENETICS
Recurrent rearrangements of the short arm of chromosome 17 (p13.2) have been found in approximately 63% of cases of ABC [7]. This locus belongs to the ubiquitin-specific protease 6 (USP6), also known as Tre-2. Through promoter swapping, USP6 contributes its entire coding sequence as the 3' portion, and its transcription is substantially enhanced by replaceable 5' partner genes juxtaposed to the untranslated regulatory element of USP6 [16]. As a result, the gene induces the production of matrix metalloproteinase activity via nuclear factor-kB. The most common fusion partner for the USP6 gene is CDH11 (about 30%); however, other genes such as TRAP150 (THRAP3), ZNF9 (CNBP), OMD, COL1A1, RUNX2, PAFAH1B1, CTNNB1, SEC31A, E1F1, FOSL2, STAT3, USP9X, ASAP1, FAT1, SAR1A, TNC, SPARC have been reported [17-19]. Rare cases of an unusually aggressive ABC with soft tissue extension and RUNX::USP6 fusion is also reported [20]. Rearrangement of the USP6 gene can be detected by fluorescence in-situ hybridization or fusion panel analysis such as targeted RNA sequencing.
Rearrangement of the USP6 gene has also been found in other soft tissue neoplasms; some with a limited capacity of bone formation, mimicking sarcomas; however, with limited growth potential and non-aggressive clinical course. These entities include nodular fasciitis, cellular fibroma of tendon sheath, myositis ossificans, and fibro-osseous pseudo tumor of digit. This common finding suggests that these neoplasms might belong to a spectrum of disease processes that can best be referred to as USP6-associated neoplasms [16,21-24].
DIFFERENTIAL DIAGNOSIS
It is important to differentiate primary ABC from lesions with ABC-like changes. Such changes are more common in giant cell tumors of bone, fibrous dysplasia, chondroblastoma, osteoblastoma, and even osteosarcoma [4].
Radiographic findings often aid in narrowing down the differential diagnoses. As a general rule, any epiphyseal or diaphyseal-based lesion raises the possibility of ABC-like changes more than the primary ABC. For example, a diaphyseal lesion with multiple cysts and fluid-fluid levels indicates the possibility of telangiectatic osteosarcoma. The cystic epiphyseal-based lesions suggest the ABC-like changes in an underlying giant cell tumor of bone or chondroblastoma, depending on the patient’s skeletal maturity.
In cases of giant cell tumor of bone with secondary ABC changes, immunohistochemical stain for H3F3A G34W (or other histone markers) can aid in diagnosis. The mononuclear cells of the giant cell tumor show nuclear immunoreactivity for the histone markers; however, the primary ABC does not demonstrate such a finding.
Distinction from telangiectatic osteosarcoma can be challenging due to some overlapping clinical and radiographic features. Unlike ABC, telangiectatic osteosarcoma shows highly atypical and anaplastic cells within the stroma, frequent atypical mitoses, and sometimes necrosis. Osteoid matrix production is minimal in these lesions. ABC shows frequent rearrangement of the USP6 gene; however, telangiectatic osteosarcoma does not show such findings. There is no specific immunohistochemical stain that aids in the differential diagnosis of these entities.
Central giant cell granuloma involves the gnathic bones and mimics “solid ABC.” It is usually solid with no or minimal cystic components. Cytologic atypia and necrosis are also not common. Unlike ABC, they lack rearrangement of the USP6 gene [25].
TREATMENT AND PROGNOSIS
Although the overall prognosis of ABC is good, the goal of any treatment modality is to slow down the disease progression, symptom relief, and fixation or prevention of pathologic fracture. En-bloc resection, although produces the least rate of disease recurrence [26,27], is not commonly performed due to an increased rate of functional impairment and morbidity [28]. Curettage with or without bone grafting is more commonly performed especially in anatomic locations amenable to surgical intervention.
Other therapeutic modalities including percutaneous doxycycline injection [29], arterial embolization, steroid or calcitonin injection, bisphosphonates, and RANKL inhibitors have been shown to be effective in the treatment of ABC in special clinical settings [30-35].
Local recurrence is seen in up to 1/3 of the cases, especially within the few months after the initial treatment, however, it is very rare after 2 years. Young age and open growth plates are also associated with an increased risk of local recurrence [36]. Rare cases of ABC with metastatic disease have also been reported [37].
CONCLUSION
Proper characterization of primary ABC of bone from ABC-like changes in other benign and malignant diseases requires the correlation of radiographic and pathologic findings. In challenging cases, molecular studies to identify the rearrangement of the USP6 gene will aid in diagnosis.
Notes
Ethics Statement
Not applicable.
Availability of Data and Material
Data sharing not applicable to this article as no datasets were generated or analyzed during the study.
Code Availability
Not applicable.
Author contributions
Conceptualization: JDR, EN. Investigation: EN. Project administration: EN. Supervision: JDR. Visualization: EN, JDR. Writing—original draft: EN. Writing—review & editing: EN, JDR. Approval of final manuscript: EN, JDR.
Conflicts of Interest
The authors declare that they have no potential conflicts of interest.
Funding Statement
No funding to declare.