Parathyromatosis: Critical Diagnosis Regarding Surgery and Pathologic Evaluation
Article information
Abstract
Parathyromatosis, in which several nodules of hyperfunctioning parathyroid tissue form in the neck and mediastinum, is a rare cause of recurrent hyperparathyroidism. However, there are some theories regarding the origin of parathyromatosis, and seeding after rupture of the parathyroid gland capsule during surgical removal of a parathyroid lesions is the most regarded one. Herein, we report a 41-year-old man who presented with multiple parathyroid nodules in and around the left thyroid lobe 5 years after parathyroid surgery for secondary hyperparathyroidism that was finally diagnosed as parathyromatosis. We discuss the differential diagnosis of parathyromatosis from other parathyroid tumors, particularly from parathyroid carcinoma, which is important in the management of a suspected lesion.
Parathyromatosis is a rare cause of recurrent hyperparathyroidism, in which several nodules of hyperfunctioning parathyroid tissue form in the neck and mediastinum.1 Two theories for the development of parathyromatosis have been suggested. The first speculates that parathyromatosis is the result of improper handling of the glands during surgical extirpation, whereas the second defines it as parathyroid gland left behind during ontogenesis that develops under physiological pressure into hyperfunctioning nests of tissue.2 Additionally, some authors have proposed a third theory that regards parathyromatosis as a low grade parathyroid malignancy.3 Parathyromatosis most commonly occurs as a secondary phenomenon subsequent to neck surgery for hyperparathyroidism.4
We report a patient with parathyromatosis showing atypical histological features short of definite malignancy, who presented with recurrent hyperparathyroidism 5 years after a parathyroidectomy for secondary hyperparathyroidism.
CASE REPORT
A 41-year-old man with chronic renal failure and secondary hyperparathyroidism, with a serum calcium level of 11 mg/dL and a parathyroid hormone (PTH) level of 1,520 pg/dL, underwent a two-gland total parathyroidectomy. A histopathological examination of the superior and inferior left parathyroid glands revealed hyperplastic nodular parathyroid tissue. After the first operation, his serum calcium and PTH levels decreased to 7.3 mg/dL and 211 pg/mL, respectively. Hyperparathyroidism (PTH, 1,814 pg/mL) and hypercalcemia (calcium, 10.7 to 11.1 mg/dL) recurred 5 years later, and multiple parathyroid nodules were detected on neck ultrasound imaging (USG), which were 8-20 mm in diameter in and around the left thyroid lobe. No pathological lymph node was detected on neck USG.
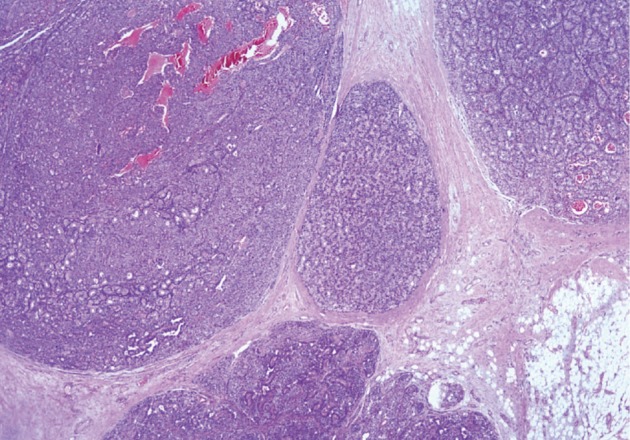
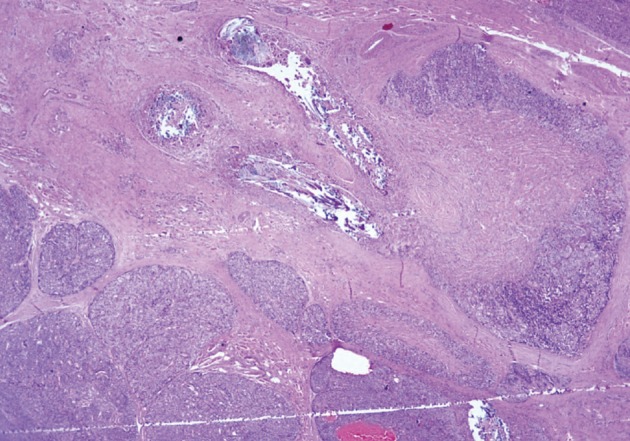
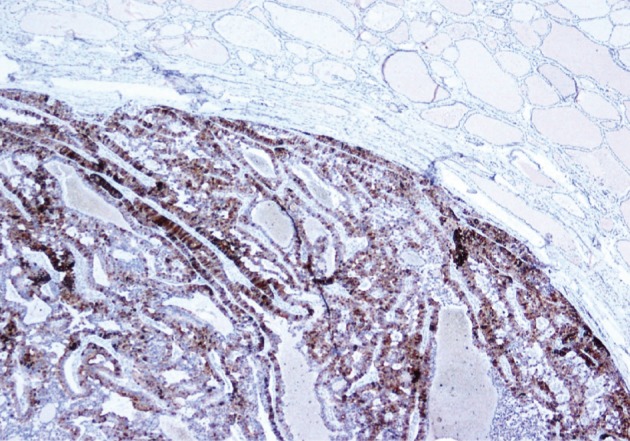
He underwent surgery again, and the left thyroid lobe with surrounding masses was resected en bloc. The resected tissue showed nodules of varying sizes with smooth edges in the periphery of the left thyroid gland and in the skeletal muscle extending into the thyroid gland. Histopathologically, there were nodular parathyroid tissues of varying cellularity and configurations (Fig. 1). The nodules were round with smooth noninfiltrative borders. In one field, suture material was evident between the nodules, which was surrounded by multinucleated giant cells and indicating the previous operation (Fig. 2). Most of the nodules were composed of uniform cells with clear to amphophilic cytoplasm and small dark nuclei, resembling chief cells (Fig. 3). Nuclear enlargement and a prominent nucleolus were detected in only a few cells of some nodules (Fig. 4). The mitotic rate was 1-2 in 50 high power fields (HPF) but was as high as 7 in 50 HPF in a limited number of fields. Atypical mitoses were not present. No desmoplastic or inflammatory response in or around the nodules was observed, and no vascular invasion was detected. PTH showed a diffuse immunohistochemical staining pattern (Fig. 5) whereas galectin-3 showed focal positivity (Fig. 6) in the parathyroid nodules. The Ki-67 proliferation index was low at 1-2% in most of the fields but was as high as 10% in some fields (Fig. 7).
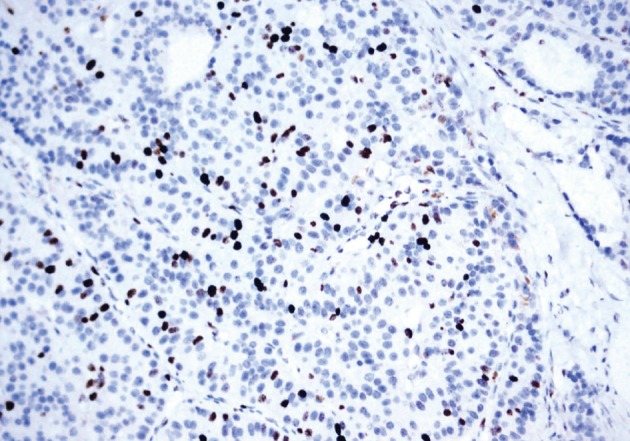
The Ki-67 proliferation index is low at 1-2% in most of the fields but was as high as 10% in some fields.
His postoperative serum PTH level decreased to 11.5 pg/mL, and his serum calcium level ranged from 6-9.4 mg/dL at different times. The patient was free of recurrence and metastatic disease at the 12 months follow-up.
DISCUSSION
Parathyromatosis may arise primarily or more commonly secondarily in patients who have had prior neck surgery for secondary hyperparathyroidism.4 Two theories have been suggested for the development of parathyromatosis. The first speculates that parathyromatosis is the result of improper handling of the glands during surgical extirpation, whereas the second defines it as parathyroid gland left behind during ontogenesis that developed under physiological pressure into hyperfunctioning nests of tissue.2 Additionally some authors have proposed a third theory that regards parathyromatosis as a low grade parathyroid malignancy.3 However this theory has not been accepted by further study.
Parathyroid carcinoma is the main entity to be excluded clinicoradiologically and histopathologically in the differential diagnosis of parathyromatosis. Parathyroid carcinoma is most often associated with primary hyperparathyroidism. Patients with parathyroid carcinoma usually have more profound hypercalcemia and metastases.1 Fernandez-Ranvier et al.1 found that calcium levels were significantly higher (≥14 mg/dL) in patients with parathyroid carcinomas than those in patients with parathyromatosis.
A differential diagnosis between parathyroid carcinoma and parathyromatosis can be challenging intraoperatively. Parathyroid carcinomas usually present as solitary tumors, whereas parathyromatosis present as small and numerous nodules. In contrast, parathyromatosis is often surrounded by adherent fibrous tissue due to prior surgery, which gives the impression of a parathyroid carcinoma. Intraoperative findings can be challenging for the surgeon who tries to distinguish parathyromatosis from parathyroid carcinoma. Some authors recommend treating all such patients as if their lesions are cancerous, because occult tumors can be present in both conditions.1,5
Histopathological criteria to diagnose parathyroid carcinoma should include a trabecular growth pattern, thick fibrous trabecula, mitotic figures (>1/10 HPF), capsular invasion, vascular invasion, and lymph node metastases or distant metastasis.1 In contrast to adenomas and primary parathyroid carcinomas, the real capsule is absent in the nests of the parathyroid tissue in patients with parathyromatosis. In the present case, although high mitotic rate focal areas (7/50 HPF), a trabecular growth pattern, and nuclear pleomorphism were present, these did not provide a definitive diagnosis of parathyroid carcinoma alone. Furthermore, all of these features that support the diagnosis of parathyroid carcinoma were also described in patients with parathyromatosis by Fernandez-Ranvier et al.1 Additionally, vascular invasion, lymph node metastases, or distant metastasis, which are the reliable criteria for malignancy of endocrine neoplasms, were not present in our case.
Some immunohistochemical studies have been conducted to distinguish parathyroid carcinoma from benign masses. Loss of parafibromin expression, Rb expression, and galectin-3 overexpression distinguish parathyroid carcinoma from other parathyroid tumors.6 As in other studies that documented galectin-3 expression in some parathyroid adenomas and hyperplastic parathyroid glands,6-8 galectin-3 overexpression occurred in our case. However, this finding alone does not permit a malignant diagnosis.
In conclusion, parathyromatosis must be considered in the differential diagnosis of a parathyroid tumor during clinical, radiological, and pathological evaluations. It is critical for the differential diagnosis to evaluate a tumor for any features that support a parathyroid carcinoma (infiltrative borders, lymphovascular invasion, and solitary nodules) or for the presence of metastatic disease. Patient history should also be reviewed for a previous parathyroid surgery.
Notes
No potential conflict of interest relevant to this article was reported.