Collision of Adenocarcinoma and Schwannoma of the Stomach: A Case Report
Article information
Abstract
The simultaneous occurrence of an adenocarcinoma and schwannoma is extremely rare in the stomach, and only one such case has been previously reported, which presented as two separate masses. Indeed, the collision of these tumors has never been reported. We report the case of a 61-year-old male patient who was diagnosed with the synchronous development of a schwannoma and advanced mucinous adenocarcinoma of the stomach, in which the carcinoma cells focally invaded the schwannoma.
The synchronous occurrence of an adenocarcinoma and mesenchymal tumor is uncommon in the stomach1 and only 22 cases of synchronous gastric adenocarcinomas and gastrointestinal stromal tumors (GISTs) have been reported.1-7 The co-existence of an adenocarcinoma and schwannoma in the stomach is extremely rare and only one case has been previously reported, which presented as two separate masses.1 Herein we report the first case of a collision tumor involving an adenocarcinoma and schwannoma in the stomach.
CASE REPORT
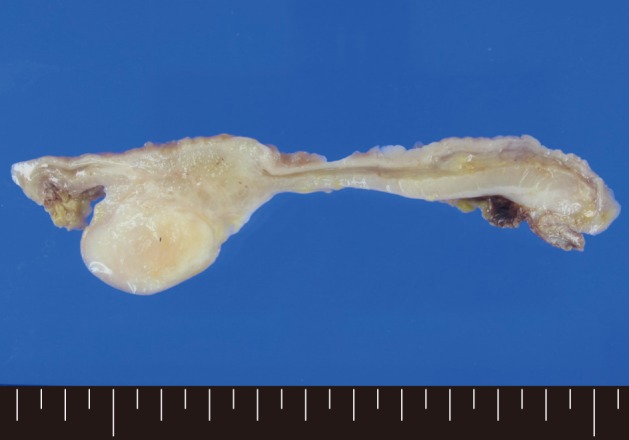
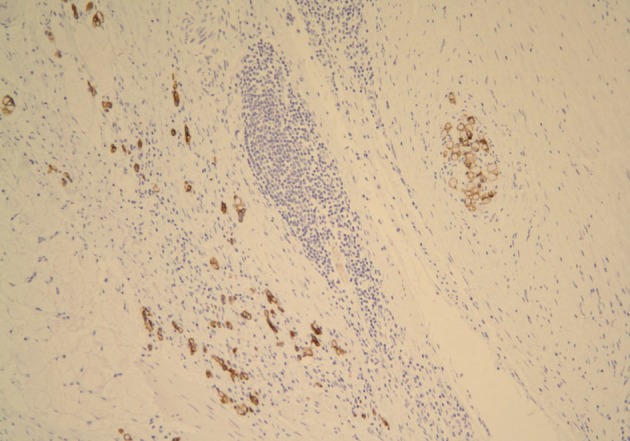
A 61-year-old male was admitted to our hospital for evaluation of melena of several days duration. He had a history of an acute myocardial infarction six years ago. Abdominal computed tomography revealed diffuse concentric wall thickening of the stomach, suggesting advanced cancer, with enlargement of multiple lymph nodes (the largest one was 2.8 cm in size and located along the gastric body of the lesser curvature directly abutting or focally invading the primary wall thickening). A distal gastrectomy was performed. The mucosa revealed an ill-defined ulcerative mass (3×2.5 cm) on the anterior wall of the body, which involved the entire thickness of the stomach. A white-gray, solid, firm mural mass (2.8×2.3 cm) was present which abutted the ulcerative mass (Fig. 1). Histologically, the main mass was a mucinous adenocarcinoma extending to the perigastric tissue. An abutting mural mass was located in the proper muscle layer consisting of vaguely whirling benign spindle cells with a peripheral lymphoid cuff (Fig. 2). Foci of loosely textured areas were also seen. Neither nuclear palisading nor Verocay bodies were found. These spindle cells were diffusely and strongly positive for S100 protein (Fig. 3) and glial fibrillary acidic protein (GFAP), but negative for c-kit, CD34, smooth muscle actin, desmin, and calretinin. Details of antibodies for immunohistochemistry are shown in Table 1. Thus, the histologic findings of the mural mass were consistent with a schwannoma. Focally, carcinoma cells infiltrated the schwannoma (Fig. 4). All 61 of the dissected lymph nodes were free of tumor.
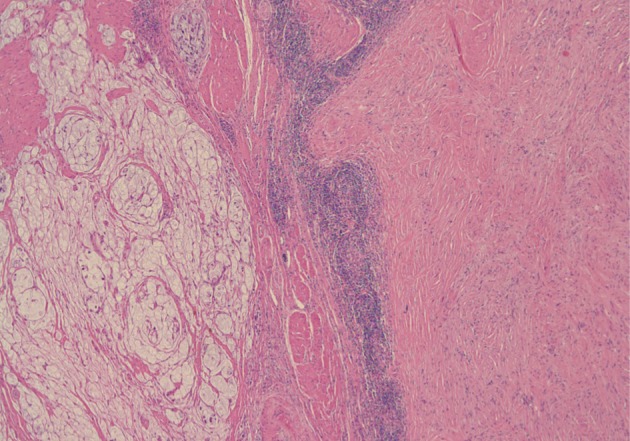
Histologically, the main mass is a mucinous adenocarcinoma and the abutting mural mass is composed of benign spindle cells with a peripheral lymphoid cuff.

Spindle cells of the mural mass are positive for S100, but negative for c-Kit and cytokeratin (immunostain for S100).
DISCUSSION
Gastric adenocarcinomas account for approximately 95% of all malignant gastric tumors.8 Gastric adenocarcinomas may co-exist with synchronous tumors of a different histologic type,2 specifically, gastric adenocarcinomas co-exist most commonly with lymphomas and less commonly with carcinoid tumors.2 The synchronous occurrence of adenocarcinomas and mesenchymal tumors is uncommon in the stomach. The majority of synchronous gastric epithelial and mesenchymal tumors are adenocarcinomas and GISTs,1 and only 22 cases of synchronous gastric adenocarcinomas and GISTs have been reported.1-7 Moreover, co-existence of adenocarcinomas and schwannomas in the stomach is extremely rare and only one case has been previously reported.1
Schwannomas are rare in the stomach, occurring with a frequency similar to leiomyomas.9 Schwannomas are benign neoplasms displaying Schwann cell differentiation.10 These tumors form intramural masses 2-10 cm in size1 and most involve the submucosa and muscularis propria.10 Schwannomas can present in a manner similar to GISTs including gross features.9 Histologically, the typical features of schwannomas included a relatively circumscribed mass, often partly surrounded by patches of lymphoid infiltration. Schwannomas are composed of variably organized tumor cells, often arranged in a microtrabecular pattern in a collagenous background. Focal nuclear atypia is common, but mitotic activity only exceeds 5 per 50 high power fields in exceptional cases.9 Immunohistochemically, gastric schwannomas are positive for S100 protein and usually GFAP, but negative for c-Kit and CD34.9
Lesions classified as gastric schwannomas differ from conventional somatic soft tissue schwannomas histologically by having peripheral lymphoid cuffs lacking a fibrous capsule and rarely showing degenerative changes.10 Nuclear palisading is uncommon in gastric schwannomas.11 Gastric schwannomas lack alterations in the NF2 gene, which are found in many sporadic conventional schwannomas from other sites, and do not express calretinin.10
The differential diagnosis of the spindle cell lesion in our case includes schwannomas, GISTs, and smooth muscle tumors. Schwannomas can be distinguished from GISTs because GISTs typically show c-Kit and CD34 labeling and lack S100 protein expression.9,10 Moreover, GISTs do not have an inflammatory cuff,10 which is a peculiar feature of gastric schwannomas. Smooth muscle tumors do not have a lymphoid cuff and express smooth muscle markers including desmin, actin, and calponin, which differ from schwannomas.10 Our case can be differentiated from neurofibroma, another type of peripheral nerve sheath tumor, because the tumor cells showed uniform intense immunostaining for S100 protein.
In our case, the mural mass consisted of benign spindle cells, which were diffusely and strongly positive for S100 protein and GFAP, but negative for c-Kit, CD34, smooth muscle actin, and desmin. With characteristic histologic and immunohistochemical findings, the spindle cell lesion was diagnosed as a gastric schwannoma.
In most of the reported cases of synchronous gastric adenocarcinomas and GISTs, gastric adenocarcinomas coexisted with GIST in a different part of the stomach, and the pre-operative biopsy fragments showed adenocarcinomas only and GISTs were detected in following laparotomy and examination of the resected specimens.2 However, tumor cells of different histologic types rarely coexist in the same location, and form a collision tumor in the stomach.2,5,12,13 One case previously mentioned the synchronous occurrence of a primary adenocarcinoma and schwannoma of the stomach that presented as two separate masses. In our case, however, a schwannoma was detected on the pre-operative computed tomography and thought to be an enlarged lymph node near the main mass. The surgical specimen revealed that the main mass, an advanced mucinous adenocarcinoma, was present adjacent to the mural mass, a schwannoma, and the carcinoma cells focally infiltrated into the schwannoma. Therefore, the present case is a collision tumor of a schwannoma and adenocarcinoma occurring in the stomach.
The simultaneous finding of epithelial and stromal gastric tumors raises the questions of whether or not such an occurrence is a simple incidental association or the two lesions are connected by a causal relationship. Various hypotheses have been proposed regarding the simultaneous development of GISTs and adenocarcinomas. Coincidence alone could easily account for such an association, particularly in countries that exhibit a high incidence of gastric cancer.2 In one retrospective study, gastric adenocarcinomas were shown to harbor a synchronous GIST with an incidence of 0.25% (5/2,010 cases).14 In another study involving incidental gastric GISTs, microscopic GISTs had an incidence of 35 of 100 stomachs (35%).15 Therefore, it is possible that gastric adenocarcinomas and schwannomas of the present case can simply co-exist in the same stomach because of the high incidence of gastric cancer in Korea.
The possibility that gene mutations might underlie tumor predisposition in patients harboring a double gastric neoplasia cannot be theoretically discarded. An interesting hypothesis is that a single carcinogenic agent might interact with two neighboring tissues, inducing the development of tumors of different histologic types in the same organ.2 Experimental evidences for this possibility are as follows: first, N-methyl-N'-nitro-N-nitrosoguanidine induces the development of gastric adenocarcinomas in rats, but, when combined with agents that alter gastric mucosal barrier, leiomyosarcomas develop in conjunction with epithelial tumors. Second, whereas administration of 9,10-dimethyl-1,2-benzanthracene (DMBA) alone induces the development of adenocarcinoma, treatment with DMBA and cellophane plate causes the induction of gastric sarcoma.2 Rare examples of collision tumors consisting of a gastric adenocarcinoma and a primary GIST also suggest that these two types of tumors occur in response to a single carcinogenic agent.8 Although there are no reports of same carcinogenic agent inducing gastric adenocarcinoma and schwannoma, our case might support that possibility, because carcinoma cells focally infiltrated and intermingled with the schwannoma forming a collision tumor.
Irrespective of the origin, the co-existence of gastric mesenchymal tumors with epithelial tumors has increased in recent years. Therefore, in any case of a GIST or gastrointestinal adenocarcinoma, the surgeon should be alert to a possible co-existing tumor with a different histologic origin.16 Herein we report the first case of a collision tumor consisting of an adenocarcinoma and schwannoma in the stomach, which raises the question that a single carcinogenic agent might interact with two neighboring tissues resulting in two types of tumors.
Acknowledgments
The present research was conducted by the research fund of Dankook University in 2010.
Notes
No potential conflict of interest relevant to this article was reported.