Fine Needle Aspiration Cytology of Thyroid Follicular Neoplasm: Cytohistologic Correlation and Accuracy
Article information
Abstract
Background
This study evaluated the accuracy of fine needle aspiration cytology (FNAC) in cases of follicular neoplasm (FN) on the basis of histologic diagnosis, and reviewed the cytologic findings of FN according to the FNAC.
Methods
Among the 66 cases diagnosed with thyroid FN by FNAC during the 7-year period from 2003 to 2009, 36 cases that had undergone thyroid surgery were available for review. Cytologic diagnosis was compared with the histologic diagnosis of each case.
Results
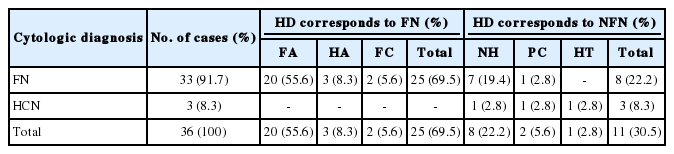
Among the 36 cases with a cytologic diagnosis of thyroid FN, histologic diagnosis was as follows: 20 follicular adenomas (55.6%), 3 Hurthle cell adenomas (8.3%), 2 follicular carcinomas (5.6%), 8 nodular goiters (22.2%), 2 papillary carcinomas (5.6%), and 1 Hashimoto's thyroiditis (2.8%), resulting in a diagnostic accuracy of FNAC for thyroid FN of 69.5%.
Conclusions
This study shows that FNAC for thyroid FN is a useful primary screening method because when FN is diagnosed by FNAC, the rate of FN histologic diagnosis is relatively high, however, adequate sampling and experience is a prerequisite for this procedure.
Recently, as the use of ultrasonographic examination has increased, the possibility of incidental findings of thyroid nodules has also increased. In Korea, the prevalence of thyroid nodules detected by an ultrasonogram is approximately 19-67%.1,2 The fine needle aspiration cytology (FNAC) of the thyroid gland is an important and definitive method for the diagnosis of thyroid nodules.3,4 Follicular neoplasm (FN) and Hurthle cell neoplasm (HCN) are relatively rare diseases and their cytologic diagnosis is difficult when compared with papillary carcinoma (PC) which shows a cytologic accuracy of more than 90%. In addition, cytologic differentiation between benign and malignant tumors is not possible in FN and HCN cases. When the histologic correlation is made, the diagnostic accuracy and predictive malignancy rate of FN and HCN are much lower compared with PC. According to the guidelines for the treatment of thyroid nodules provided by the Korean Thyroid Association, surgery is recommended for patients when FN or HCN is diagnosed by FNAC because the possibility of malignancy in this case is not known until the histologic diagnosis is made from the lobectomy or total thyroidectomy specimen.1,3 In contrast, guidelines for the treatment of PCs are relatively well established according to the categories of cytologic diagnosis. FN and HCN are still rare FNAC findings. Follicular carcinoma (FC) comprises approximately 5% of thyroid cancers,5 and because the number of FN cases is limited and FC cannot be distinguished from benign follicular adenoma (FA) on the basis of cytologic findings, describing FN cytologically in an ambiguous manner may be inevitable. Therefore, more cytologic information regarding FN and HCN are required not only for the cytologic diagnosis but also for the development of appropriate treatment guidelines.
In this study we evaluated the FNAC accuracy in FN cases based on the histologic diagnosis and investigated the cytologic findings to increase the probability of a correct cytologic FN diagnosis.
MATERIALS AND METHODS
Among the 66 cases that had been diagnosed with thyroid FN by FNAC during the 7-year period from 2003 to 2009, 36 cases that underwent thyroid surgery were available for review. FNAC was performed under ultrasonographic guidance in all cases. We reviewed the cytology slides on the basis of representative cytologic FN findings such as abundance of follicular epithelial cells, presence of microfollicular structures, abundant cell crowding, abundant dispersed isolated cells, homogenous nuclear morphology, lack of nuclear grooves, lack of colloid material and lack of macrophages with reference to previous reports and the Bethesda system for reporting thyroid cytology.2,4,6,7 Regarding HCN, we looked only for the characteristic findings of Hurthle cells such as abundant finely granular cytoplasms enlarged, central or eccentrically located, round nucleus, prominent nucleolus, small cells with high nuclear/cytoplasmic ratio (small cell dysplasia), and large cells with at least 2× variability in nuclear size (large-cell dysplasia).7 However, for the cytologic review we applied the same cytologic standards as in FN. After slide review, cytologic diagnosis was compared with the histologic diagnosis of each case. We selected the cases which satisfied the standard adequacy criteria for interpreting thyroid cytology. The standard adequacy criteria is the presence of at least 6 groups of follicular cells in total on stained smears, with a minimum of 10 cells in each group.8 The slides used in this study were made by a conventional method and reviewed by 2 pathologists according to the guidelines provided in the Bethesda system.7 For confirmation of statistical significance the chi-square test was performed using SAS ver. 8 (SAS Inc., Cary, NC, USA). In all statistical analyses, a p<0.05 was considered significant. This study's protocol was approved by the Institutional Review Board of our hospital (no. VC11SISI0147).
RESULTS
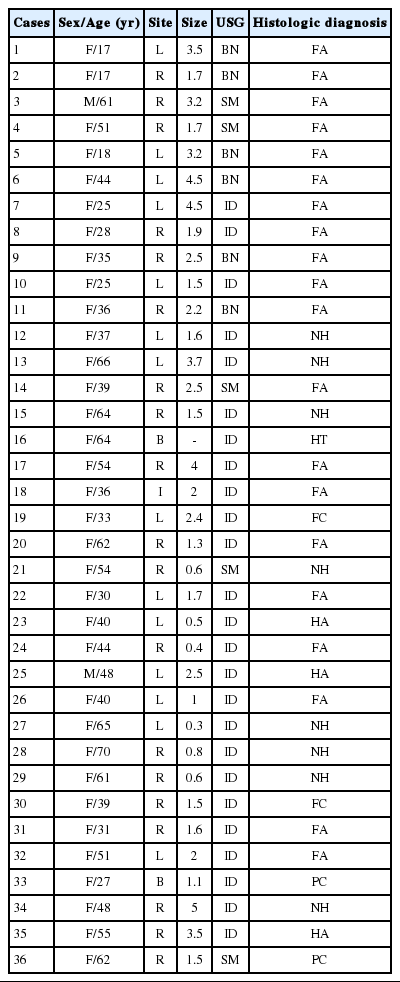
A majority of the cases were female and only 2 cases were male. Ultrasonographic findings showed that 6 cases were benign, 5 cases were suspicious for malignancy, and 25 cases were indeterminate (Table 1). Among the 36 cases in which surgery was performed, 33 (91.7%) were diagnosed as FN and 3 (8.3%) were diagnosed as HCN in the preoperative FNAC. Of the 33 cases, 20 (55.6%) were diagnosed as FA, 3 (8.3%) were Hurthle cell adenoma (HCA), 2 (5.6%) were FC. A total of 25 cases (69.5%) were consistent with FN and the other cases included 8 (22.2%) nodular goiters, 2 (5.6%) PCs, and 1 (2.8%) Hashimoto's thyroiditis. All 3 cases which were cytologically diagnosed as HCN were proven histologically to be non-FN. Thus, the FNAC diagnostic accuracy for FN was 69.5% (Table 2). Regarding the clinicopathologic characteristics of the 25 cases which were diagnosed as FN, 23 were female and 2 were male. The age varied from 17 to 62 years and the average age was 38.4 years. The tumor sizes evaluated from the lobectomy or total thyroidectomy specimens ranged from 0.4 cm to 4.5 cm with an average size of 2.29 cm. The lesion sites included the right lobe (13 cases), left lobe (11 cases), and isthmus (1 case). Histologic subtypes were as follows: microfollicular (19 cases), normofollicular (1 case), oncocytic (3 cases), microfollicular with trabecular (1 case), and microfollicular with papillary hyperplasia (1 case) (Table 1).
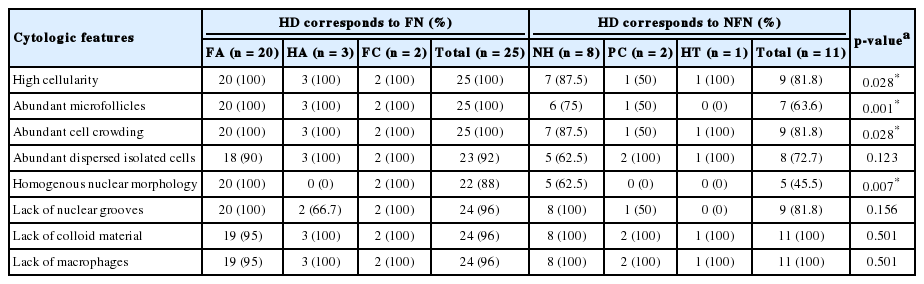
These cases had generally sufficient cellularity for a proper diagnosis, and the majority showed the characteristic cytologic findings including abundance of follicular epithelial cells, presence of microfollicular structures, abundant cell crowding, abundant dispersed isolated cells, homogenous nuclear morphology, lack of nuclear grooves, lack of colloid material and lack of macrophages (Fig. 1). Among these cytologic features, high cellularity, abundant microfollicles, abundant cell crowding, and homogenous nuclear morphology were especially important features for the FN cytologic diagnosis (Table 3).
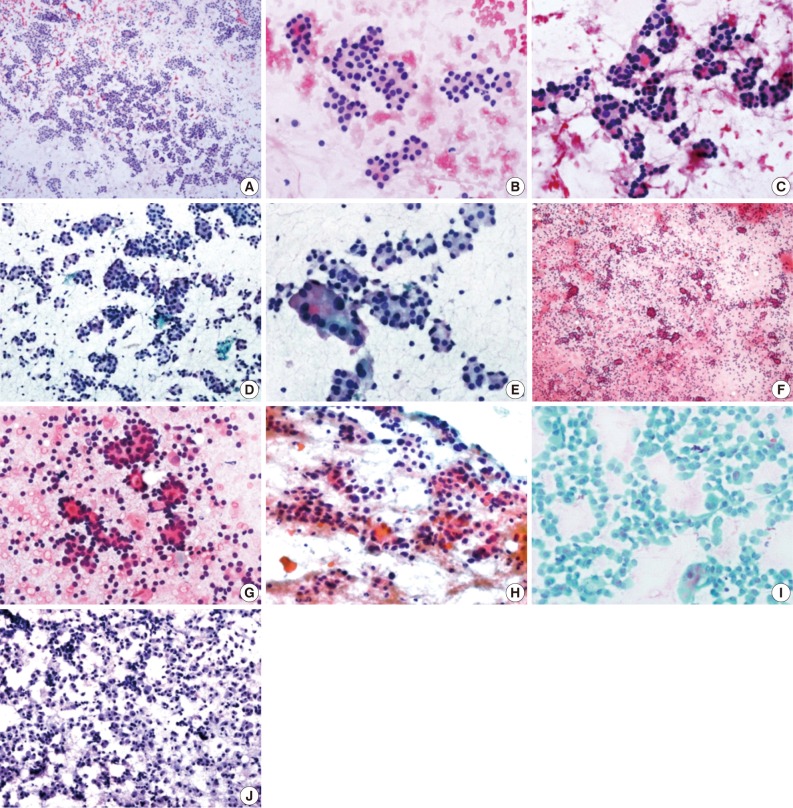
Cytologic features of follicular adenoma (FA). (A) Low power view of fine needle aspiration cytology (FNAC) shows high cellularity composed of abundant microfollicles. (B) The crowded follicular cells have homogenous nuclear morphology composed of round nuclei, evenly dispersed, granular chromatin and faint nucleoli. (C) Microfollicles contain small amounts of colloid. (D) Hurthle cell type. The aspirate is very cellular and consists almost exclusively of Hurthle cells in small crowded arrangements. (E) Hurthle cell type. High-power view shows an abundant, finely granular cytoplasms and enlarged, variable sized, eccentrically located nucleus. (F) FNAC of follicular carcinoma (FC) confirmed by histologic diagnosis shows abundant syncytial clusters and small follicles similar to FA. (G) High-power view of FC shows more predominantly abundant, dispersed, isolated cells than FA in both cases. (H) FNAC (cytological misdiagnosis of Hurthle cell neoplasm [HCN]) of HCN with Hurthle cell change confirmed by histologic diagnosis shows abundant syncytial clusters composed exclusively of Hurthle cells. (I) Papillary carcinoma, oncocytic variant misdiagnosed as FA, with Hurthle cell type as determined by FNAC, showing definitive nuclear inclusion and mitosis. (J) Hashimoto's thyroiditis confirmed by histologic diagnosis misdiagnosed as FA, with Hurthle cell type as determined by FNAC, showing abundant Hurthle cells and a few lymphocytes.
DISCUSSION
The purpose of cytologic or pathological examination reports is to provide clinicians appropriate guidelines for the treatment of patients, including surgery to the clinicians.9 In the field of cytology, several report formats have been developed for this purpose, and presently, most reports are provided using categorical terms.10 Several forms of categorical diagnosis have been introduced. Generally, they include categories of unsatisfactory, benign, suspicious for malignancy, malignancy, and some forms have additional categories such as indeterminate, atypical, or follicular neoplasia.10 FA is defined as solitary encapsulated nodules arising in an otherwise normal thyroid that lack evidence of capsular or vascular invasion.5 HCN is considered a biologically different disease entity from FN, but is reported in the same category as FN with the additional mention of the possibility of HCN.7 Traditionally, FN and HCN were ambiguously classified in cytology because of the limitations of their cytologic diagnosis. Recently, the Bethesda system for reporting thyroid cytopathology was introduced which classified FN/HCN in the independent category. The traditional category of atypia includes the cytologic findings of suspicious FN/HCN in the terminology of follicular lesion of undetermined significance.7 However, the cytologic diagnosis of FN/HCN remains clinically controversial when compared to PC, which provides effective guidelines for the clinician.11,12 Using FNAC, the diagnostic accuracy of PC is more than 90%, but the distinction between malignancy and benign in FN or HCN is impossible because surgery is mandatory for the definitive diagnosis of FC or Hurthle cell carcinoma.2,13 Several diagnostic schemes including the Bethesda system described the malignancy rate, but in the case of FN, the malignancy rate is very low when compared to suspicious PC.7
The Korean Thyroid Association introduced the revised edition of the updated guidelines for the diagnosis and management of thyroid nodules in 2010.1,3 In the revised guidelines, the FNAC results were described according to the Bethesda system. In this guideline, FNAC is recommended for nodules of more the 1.0 cm in size, nodules less than 1.0 cm in size but associated with risk factors, cystic nodules more than 2.0 cm in size, or patients with Hashimoto's thyroiditis because it is more commonly associated with PC.3,14 The revised edition also used the Bethesda system for the provision of treatment guidelines. For unsatisfactory cases, a repeat examination with follow-up or consideration of surgery is recommended. For benign cases, additional examination or treatments are not necessary. In the atypia category, because the malignancy rate is approximately 5-15%, a repeat FNAC with consideration of surgery is necessary. The category of suspicious malignancy or malignancy is indication for lobectomy or total thyroidectomy. For the FN, when the autonomic nodules are not found in the thyroid scan, lobectomy or total thyroidectomy is recommended. For the HCN, lobectomy or total thyroidectomy is recommended without a thyroid scan.1 In the FN or HCN cases, although a few cytologic findings can be helpful for the suspicion of FN/HCN, the prediction value of malignancy is relatively low.3,15 FNAC cannot provide definitive criteria for the distinction between benign and malignancy when FN/HCN is suspected.
Diagnosis of FN/HCN by FNAC is the subject of several studies from Korea and other countries. In these studies, the cytologic diagnosis rate of FN was approximately 10% and a majority of FA or FC cases confirmed by histologic diagnosis showed a previous cytological diagnosis as benign, nodular hyperplasia, and even as PC.10,12,16-20 Most pathologists do not have sufficient experience with FN/HCN. In FNAC, the most important causes of diagnostic misinterpretation were overlapping cytological features among follicular-derived lesions and inadequate/suboptimal specimens. Especially, the follicular variants of PC, FN, and adenomatous hyperplasia show overlapping cytological features making the diagnosis of FN/HCN more difficult.16,21
Although difficult, the ultimate purpose of FNAC in FN/HCN cases is to isolate FC. The present study showed the diagnostic specificity of FA including HCA was 64%, with a malignancy rate of 11% when a histologic correlation was conducted. Among the malignancies, 2 FCs and 2 PCs were identified. The malignancy rate in the present study did not reach the same level as with the Bethesda system, but PCs were not exclusively included. Therefore, PC was successfully screened by FNAC with the relevant cytological diagnosis, and the diagnostic specificity of FN by FNAC was relatively high when compared with the histologic diagnosis. Specifically, among the FNs, 2 FCs were successfully identified even though the number of cases was small. These results are well correlated with the clinical experience that lobectomy or total thyroidectomy is recommended for the cytologically suspected FN. The FN category is controversial because follicular lesions include various diseases and their differential diagnosis by FNAC is not readily made, and in particular, considerable areas of cytologically suspected FNs were identified as PCs by histologic examination.22 Because this category predicts the existence of FC to some extent and leads to surgical treatment, this category has sufficient value in FNAC.
The present study started with the selection of cytologically diagnosed FN, and cases were chosen where correlation with histologic diagnosis was available. Therefore, this was a slightly different approach when compared with preexisting methods which started with the histologically confirmed FA or FC cases and followed by analysis of their previous cytological diagnoses. However, this approach generally results in a low diagnostic FNAC rate in the case of FNs. These results can be easily verified in the above-mentioned reports. The present study shows that FNAC may be a useful method in primary FN/HCN screening of the thyroid gland, because if the FN is diagnosed by FNAC, the rate of histologic FN diagnosis is relatively high. However, adequate sampling and experience is a prerequisite for this procedure. The present study results hopefully can contribute to available data regarding FNs and be used in the future revisions and supplements of treatment guidelines for FNs.
Notes
No potential conflict of interest relevant to this article was reported.