Pleomorphic Adenoma of the Trachea: A Case Report
Article information
Although pleomorphic adenoma commonly occurs in the salivary glands of the head and neck, tracheobronchial pleomorphic adenoma is extremely rare.1 In this report, we report a case of pleomorphic adenoma of the trachea with active pulmonary tuberculosis. We performed surgical resection of pleomorphic adenoma of the trachea after management of pulmonary tuberculosis.
CASE REPORT
A 59-year-old male was referred to our hospital due to a three-month history of dyspnea on exertion. He was a current smoker with a 45-year history of smoking 2 packs of cigarettes per day. He had no past medical history except for pulmonary tuberculosis two years prior. On physical examination, the sound of his right lung was decreased with crackles but he did not have lymphadenopathy or weight loss. Laboratory findings were unremarkable except that the pulmonary function test revealed a moderately obstructive lung defect. Chest X-ray showed reactivation of pulmonary tuberculosis in the upper lung field and a round mass shadow in the mid-tracheal area (Fig. 1A). A computed tomography (CT) scan showed a 2.2 cm-sized homogenous mass originating from the left lateral inner wall of the trachea, and active pulmonary tuberculosis with cavitation in the right upper lobe and in the superior segment of the right lower lobe (Fig. 1B). Flexible bronchoscopy revealed a broad-based, polypoid mass obstructing 75% of the lumen in the left lateral wall. The mass was 5 cm above the carina, without endobronchial lesion (Fig. 2A). We decided to surgically remove the lesion after initial management for pulmonary tuberculosis since airway obstrucion could occur during the bronchoscopic procedure owing to rich vascularity on the tumor surface.
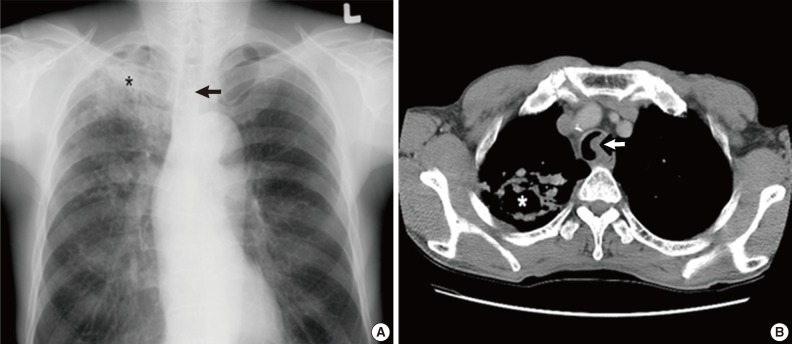
(A) Chest X-ray reveals a round mass shadow in the mid tracheal level (arrow) and tuberculosis (asterisk). (B) Computed tomographic scan of the chest shows a 2 cm-sized homogenous mass in the left lateral inner wall of the trachea (arrow) and tuberculosis with cavitation (asterisk).
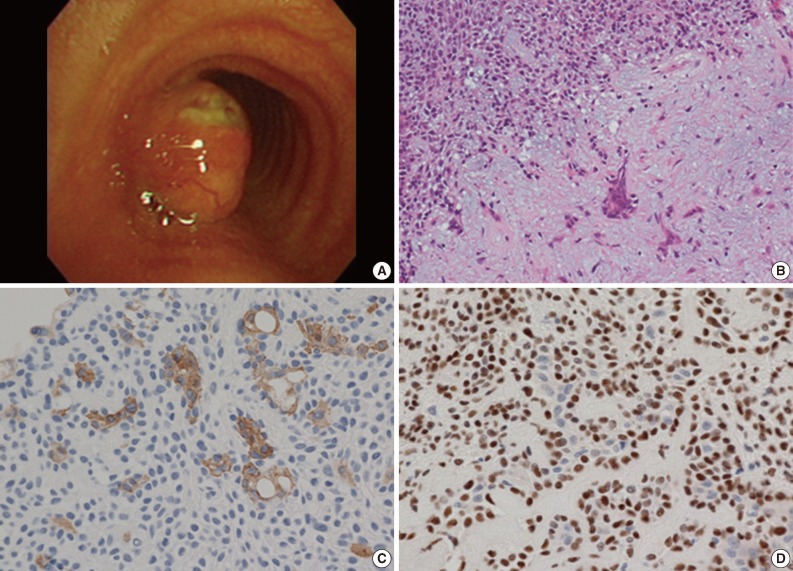
(A) Bronchoscopic finding of a broad and polypoid mass with branching blood vessels on the tumor surface with ulcer formation. (B) The tumor shows admixed epithelial component (left upper) and chodromixoidstroma (right lower) on microscopic examination. (C) The epithelial cell components are positive for cytokeratin 19. (D) The myoepithelial cell components show immunoreactivity for p63.
The patient underwent right thoracotomy with segmental resection and end-to-end anastomosis. Four tracheal rings were excised along with the tumor. The excised tumor appeared as a single well-demarcated solid, polypoid mass, measuring 2×2 cm in size. The mass was completely covered by intact mucosa without any visible sign of hemorrhage or necrosis. On microscopic examination, the mass was composed of epithelial and myoepithelial cells in the chondromyxoid stroma (Fig. 2B). There was no definitive cellular pleomorphism and mitotic figures. The surgical margins of the resected trachea were free of tumor. Immunohistochemically, the epithelial cells were positive for cytokeratin, cytokeratin 19 and epithelial membrane antigen (EMA). The myoepithelial cells were immunoreactive for cytokeratin, S100 protein, and p63 (Fig. 2C, D). Overall, these findings were suggestive of a pleomorphic adenoma involving the mid trachea. The postoperative course was uneventful, and the patient has been asymptomatic and in good health for 5 years after the surgery.
DISCUSSION
Pleomorphic adenoma is the most common tumor of the major salivary gland, rarely occurring in the lung and typically arising from the tracheal and bronchial seromucous glands.2,3 The exact definitive incidence has not been reported. The age range of patients diagnosed with pleomorphic adenoma is usually between 35 and 74 years, with no gender or age predominance,1,2 although a case has been reponted in an 8-year-old male.4 Almost half of these tumors were localized in the upper one-third of the trachea and 12% were localized in the lower one-third.5 The tumor in the present case arose from the left lateral inner wall of the lumen which was 5 cm above the carina in the mid-tracheal area. The most common symptoms are cough, sputum, dyspnea, wheeze, and stridor.1,4 Normally, because of the slow tumor growth, these respiratory signs and symptoms are misdiagnosed as asthma,5 and thus proper diagnosis may be delayed. The tumor may not be discovered on plain chest posterior-anterior view and therefore, chest CT and bronchoscopy are important in diagnosing the patient at presentation.
The histological characteristics are similar to salivary gland tumors. Microscopic findings show trabecular or islands of epithelial and myoepithelial cells in a myxoid or chondroid matrix.1 Immumohistochemical staining is helpful to diagnose pleomorphic adenoma, in which the findings are positive for the myoepithelial cell components for cytokeratin, p63 and S100 protein.5 In addition, ductal epithelial cell components are positive for cytokeratin, EMA, secretory component carcinoembryonic antigen, lysozyme, and gross cystic disease fluid protein-15. The most frequently expressed cytokeratin 19 is positive in the luminal cells of the tubular structures.6
The most difficult differential diagnosis of pleomorphic adenoma is adenoid cystic carcinoma. Tumor cell nests of adenoid cystic carcinoma are sharply separated from the stroma.7 Adenoid cystic carcinoma is an aggressive malignant tumor, therefore invasive focus, especially perineural invasion, is usually identified.
Assessing the prognosis such as biological behavior and clinical course is difficult. Histological findings associated with aggressive behavior, such as recurrence and metastasis, include infiltrative and poorly circumscribed tumor margin and high mitotic counts.1,8
There have been two reports of malignant and aggressive tumors.9,10 Thus, tumors should be considered to have malignant potential and a careful follow-up is mandatory after complete resection, as in our patient's case who is healthy 5 years postoperatively. Because the tumor rarely occurs, standard management has not been established. In general, endoscopic resection or surgical segmental resection is selected as a treatment on the basis of tumor status and physician's preference.2,5 Endoscopic removal may be used when rapid respiratory symptom improvement or hemorrhage control is required. Segmental resection is the only curative treatment for complete excision of both types of benign and malignant tumors, and decreases the possibility of lesion recurrence.
In conclusion, pleomorphic adenoma of the trachea should be included in the differential diagnosis of tracheal tumors, although the occurrence is very rare. Surgical excision is the optimal therapy for a low possibility of recurrence of pleomorphic adenoma. Future studies with additional cases are needed in order to define the clinical course, biologic behavior and prognosis of pleomorphic adenoma of the trachea.
Notes
No potential conflict of interest relevant to this article was reported.