Fine Needle Aspiration Cytology of Parathyroid Lesions
Article information
Abstract
Background
There has been an increase in the use of fine needle aspiration cytology (FNAC) for the diagnosis of parathyroid lesions (PLs). Differentiation between a thyroid lesion and a PL is not easy because of their similar features. We reviewed parathyroid aspirates in our institution and aimed to uncover trends in diagnostic criteria.
Methods
We selected 25 parathyroid aspirates (from 6 men and 19 women) confirmed surgically or immunohistochemically from 2006 to 2011.
Results
Major architectural findings of PLs include scattered naked nuclei, loose clusters, a papillary pattern with a fibrovascular core, tight clusters, and a follicular pattern. These architectures were commonly admixed with one another. Cytological features included anisokaryosis, stippled chromatin, a well-defined cell border, and oxyphilic cytoplasm. Eighteen of the 25 patients were diagnosed with PL using FNAC. Seven patients had been misdiagnosed with atypical cells (n=2), benign follicular cells (n=2), adenomatous goiter (n=2) and metastatic carcinoma (n=1) in FNAC. Using clinicoradiologic data, the sensitivity of the cytological diagnosis was 86.7%. The cytological sensitivity decreased to 50% without this information.
Conclusions
FNAC of PL is easily confused with thyroid lesions. A combination of cytological parameters and clinical data will be required to improve the diagnostic sensitivity of PLs.
Three distinct lesions can affect the parathyroid gland and cause primary hyperparathyroidism (PHPT): parathyroid hyperplasia, adenoma, and carcinoma. Parathyroid hyperplasia affects all four parathyroid glands, while parathyroid adenoma and carcinoma present as a mass affecting only a single gland.1 Among these lesions, parathyroid adenoma is the most common cause of PHPT; the only treatment option for PHPT is surgical resection of the mass.2-4 Therefore, preoperative localization is crucial in patients with PHPT.3,4 Ultrasound-guided fine needle aspiration cytology (FNAC) as well as parathyroid scanning are widely used to localize and confirm parathyroid lesion (PL) because of the convenience, cost-effectiveness, and accessibility of the head and neck.2,4-6
With increasing use of thyroid FNAC, the chances of encountering unsuspected PL are also increasing.2 Furthermore, some PLs may present as thyroid "incidentaloma," lesions accidentally found in or close to the thyroid gland. Therefore, it is important to be familiar with the cytological features of PL. However, PLs and thyroid lesions have some morphological similarities, and their differentiation is difficult. In addition, literature dealing with parathyroid cytology is scarce.1-3,6
In this study, we reviewed 25 fine-needle aspirates of PL from patients at our institution to identify the cytological features and clinicoradiologic information.
MATERIALS AND METHODS
We evaluated 25 FNAC specimens from 25 patients with well-documented cases of PL according to serum parathyroid hormone (PTH) level, immunohistochemical staining, ultrasonography, and pathology results at the Korea Cancer Center Hospital from January 2006 to May 2011. Cases that presented as a single mass were included, and those that were not confirmed by operation or immunohistochemistry were excluded. Clinicoradiologic information, such as signs or symptoms, serum PTH level, ultrasonography, and operation records, were obtained from patients' medical records. Intact PTH assay was used to ascertain serum PTH levels.
Ultrasound-guided FNAC was performed by a radiologist with a 23-gauge needle. The aspirated material was smeared onto four glass slides that were immediately fixed in 95% alcohol and subjected to Papanicolaou staining. Cell blocks were prepared by using any remaining material or by rinsing the syringes. Thyroid transcription factor-1 (TTF-1), PTH, and chromogranin A were selectively used for immunohistochemistry on cell blocks. Diagnostic terminology of "parathyroid lesion" (PL) was used if the cytological findings or immunohistochemistry results indicated a parathyroid origin. Twenty patients underwent surgery, and five patients did not. In those patients who underwent surgery, all sections of the resection specimens were fixed with 10% formalin and embedded in paraffin. In the five patients who did not receive surgery, PL was confirmed by immunohistochemical staining of cell blocks. All the slides were reviewed with a multi-headed microscope by two separate pathologists. The cytological features were categorized as follows: architectural features (scattered naked nuclei, loose clusters, a papillary pattern with a fibrovascular core, tight clusters, and a follicular pattern), nuclear features (anisokaryosis, stippled chromatin, prominent nucleoli, intranuclear pseudoinclusion, and eccentric nuclei) and other features (a well-defined cell border, oxyphilic cytoplasm, colloid-like material, and macrophages). A follicular pattern was defined as a structure resembling thyroid follicles.
RESULTS
Clinicopathologic characteristics
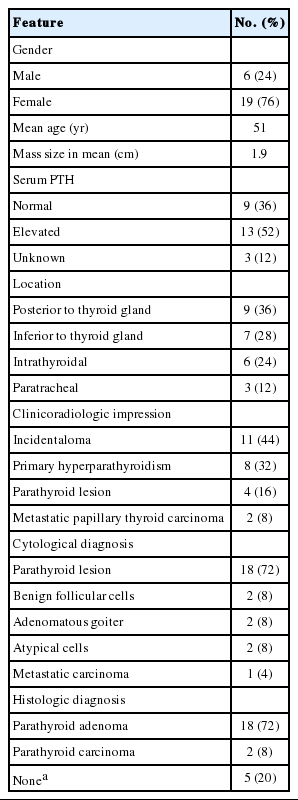
Clinicopathologic characteristics are summarized in Table 1. The patient population included six males and 19 females with an age range of 39 to 71 years (mean age, 51 years) (Table 1). Of the 25 patients, 22 were evaluated for serum PTH before the cytological examination. Thirteen of these 22 patients showed elevated serum PTH. The PL was found at the posterior surface of the thyroid gland in nine patients (36%), inferior to the thyroid gland in seven patients (28%), in the thyroid parenchyma in six patients (24%), and in the paratracheal area in three patients (12%).
The clinicoradiologic impression consisted of 11 incidentalomas (44%), eight PHPTs (32%), four PLs (16%), and two metastatic papillary thyroid carcinomas (PTC) (8%). Eighteen of the 25 cases (72%) were cytologically diagnosed as PL; the remaining seven patients were diagnosed as follows: two with benign follicular cells (8%), two with adenomatous goiter (8%), two with atypical cells (8%), and one with metastatic carcinoma (4%). When the clinicoradiologic impression suggested PL, the sensitivity of the cytological diagnosis was 86.7% (13/15). In all other cases, the sensitivity of cytological diagnosis decreased to 50% (5/10).
A minimally invasive parathyroidectomy was performed in 17 of the 20 patients who underwent surgery, and the remaining three patients received thyroid surgery or neck dissection. Eighteen of the 20 cases who received surgery were diagnosed as parathyroid adenoma, and the remaining two were determined to have parathyroid carcinoma.
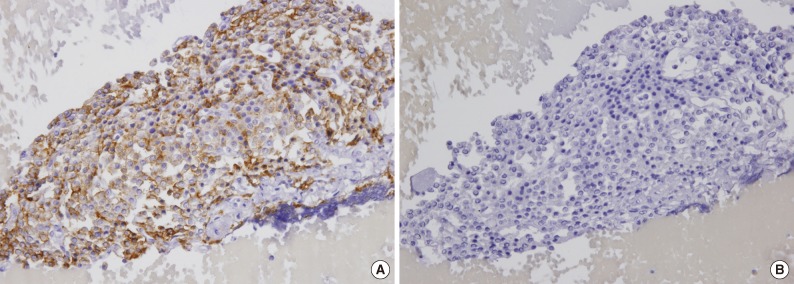
In the aspirates from the five patients who did not receive surgery, PTH was expressed in two, and the remaining three aspirates demonstrated positivity for chromogranin A. TTF-1 was negative in all four cases who were examined (Fig. 1).
Architectural features
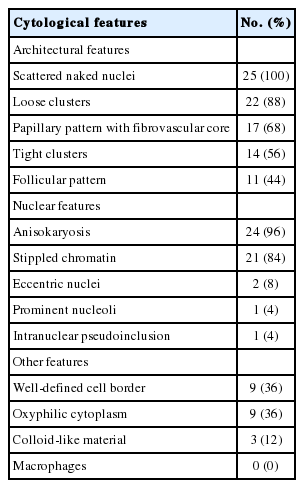
The most impressive feature of the parathyroid aspirates was the diversity of the architectural features. Most of the smears showed scattered naked nuclei (25/25, 100%) and loose clusters (22/25, 88%) in varying degrees. Other architectural features included a papillary pattern with a fibrovascular core (17/25, 68%), tight clusters (14/25, 56%), and a follicular pattern (11/25, 44%) (Table 2, Fig. 2). Each aspirate showed a combination of foregoing architectures in varying degrees. In three aspirates, the entire specimen consisted of scattered naked nuclei and closely resembled thyroiditis (3/25, 12%).
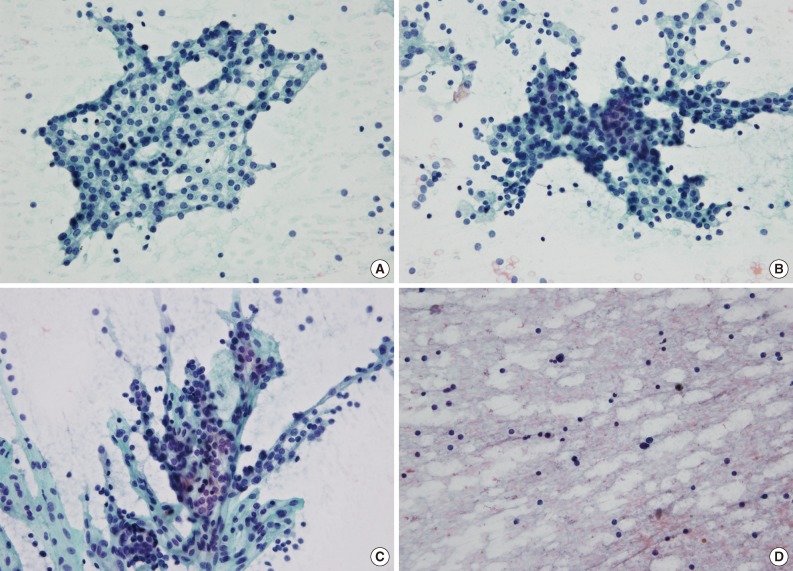
Representative cytological features of a parathyroid lesion. (A) A loose cluster demonstrates a round configuration. (B) A tight cluster shows overlapping nuclei. (C) A papillary pattern has a fibrovascular core with parathyroid cells arranged in a vascular network. (D) Smears from an aspirated intrathyroidal parathyroid lesion show a lymphoid pattern (A-D, Papanicolaou stain).
Nuclear features
Almost all nuclei were centrally located, round to oval and similar in size to red blood cells with a regular nuclear membrane. A small proportion of parathyroid cells showed mild to moderate anisokaryosis in each aspirate (24/25, 96%). The chromatin was coarse and stippled (21/25, 84%). Some cells had eccentric nuclei (2/25, 8%). Prominent nucleoli and intranuclear pseudoinclusions were only detected in one aspirate (1/25, 4%) (Table 2, Fig. 3).
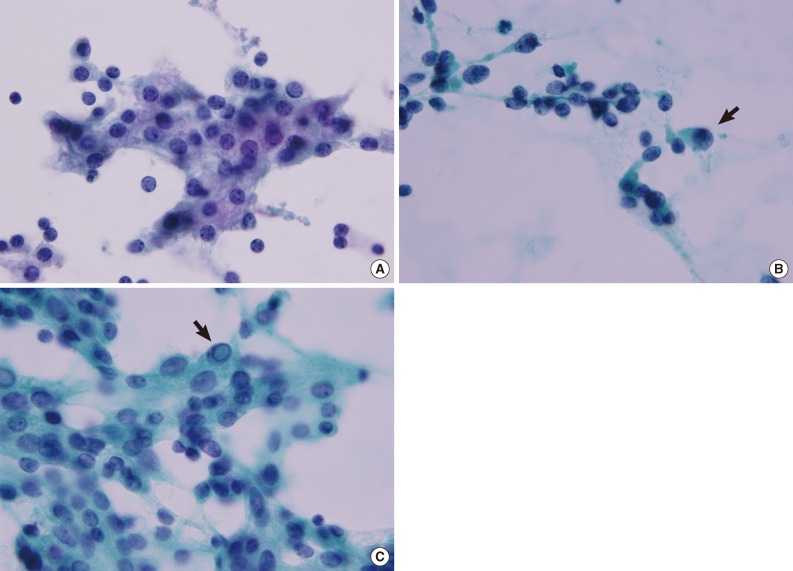
Representative nuclear features of a parathyroid lesion. (A) Nuclei are uniform and round to oval with stippled chromatin. A few cells show a well-defined cell border and an intercellular window. (B) Eccentric nuclei and anisokaryosis with a few atypical nuclei in the periphery (arrow). (C) An intranuclear pseudoinclusion has a distinct boundary resembling a shape drawn with a pencil (arrow) (A-C, Papanicolaou stain).
Other features
Many parathyroid cells had finely granular cytoplasm, and a small portion of cells revealed a well-defined cell border (9/25, 36%) and oxyphilic cytoplasm (9/25, 36%). Fragments of hyaline colloid-like material were noted in three aspirates (12%). Macrophages were not detected in our series (Table 2).
Misdiagnosis
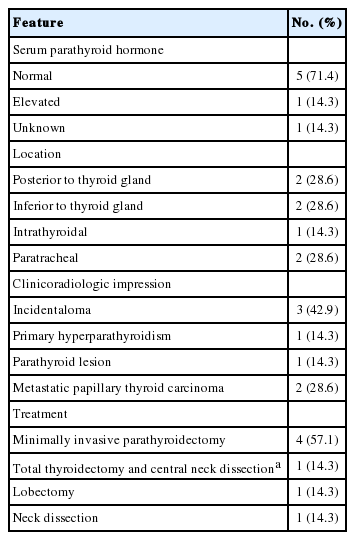
There were seven cases of misinterpreted cytology (Table 3). Two of seven cases showed elevated serum PTH (patient no. 1) or suspected PL on ultrasonography (patient no. 2). These cases were cytologically diagnosed as atypical cells and treated with minimally invasive parathyroidectomy. Another two cases (patients nos. 3 and 4) were diagnosed as adenomatous goiter; these patients underwent minimally invasive parathyroidectomy because PL was diagnosed using an intraoperative frozen biopsy. Patient no. 5 had a previous history of PTC. Clinical impression and cytological diagnosis were metastatic carcinoma, so neck dissection was performed. The dominant cytological architecture in this case consisted of tight clusters, although other cytological features of PL were present in small proportions. Patient no. 6 had a history of lobectomy due to PTC. The cytological diagnosis was benign follicular cells. The cytology slide of case no. 6 had a dominant architecture of tight clusters with scattered naked nuclei. A papillary pattern with a fibrovascular core, anisokaryosis and stippled chromatin were also found. Although PL was diagnosed using intraoperative frozen biopsy, the remaining lobe was removed by lobectomy. In patient no. 7, who had simultaneous PTC, the paratracheal mass was clinically thought to be metastatic PTC. The FNA diagnosis was benign follicular cells. The dominant architecture was loose clusters, and other cytological findings were a papillary pattern with scattered naked nuclei, tight clusters, a follicular pattern, anisokaryosis, and stippled chromatin. The patient underwent total thyroidectomy and central neck dissection.
DISCUSSION
The ability to distinguish between PL and thyroid lesions using FNAC has important clinicoradiologic significance for the treatment and quality of life of patients.6 In thyroid neoplasm, a more extensive operation, such as a lobectomy, total thyroidectomy, or neck dissection, is required, whereas PL can be selectively removed with minimal incisions.4 Therefore, pathologists need to note the cytomorphologic features of PL to ensure accurate diagnoses.
In our study, the variable architectural and cytological features of PL were well presented as follows: scattered naked nuclei, loose clusters, a papillary pattern with a fibrovascular core, tight clusters, a follicular pattern, anisokaryosis, stippled chromatin, a well-defined cell border, and oxyphilic cytoplasm. Although a few cases showed absolute predominance of one type of architecture, most cases had variable cytological features, each being mixed in varying degrees. Absher et al.2 reported that the presence of a PL may be suspected when recognizing a combination of diverse cytological features rather than a single distinct factor.
A few attempts were made to distinguish between PL and thyroid lesions by using FNAC. Absher et al.2 recommended determining certain characteristic features of PL, including cohesive cellular fragments admixed with numerous single naked nuclei, absent or inconspicuous nucleoli, round to oval uniform nuclei, smaller nuclei than thyroid nuclei, anisokaryosis, and hyperchromatic chromatin rather than finely granular chromatin. Dimashkieh and Krishnamurthy6 documented that smaller nuclei than thyroid follicular cells, bare nuclei, stippled chromatin, and a prominent vascular network with epithelial cells indicated parathyroid origin. The papillary pattern with a fibrovascular core presented in our study corresponded well with the prominent vascular network and the attached epithelial cells as well as the specific "spot of fire" hypervascularity pattern of the parathyroid gland observed on the color Doppler sonogram.4
Past studies have stated that colloid-like substances and macrophage accumulation are distinguishing cytological features of thyroid lesions from PL.1,7,8 However, recent studies have revealed that they also can be seen in PL.2,3,6 Similarly, colloid-like substances were observed in three of our cases (12%), even though macrophages were not detected.
There was one case with intranuclear pseudoinclusion, which postoperatively proved to be a parathyroid carcinoma. Intranuclear pseudoinclusion is a well-known cytological feature of PTC. However, intranuclear pseudoinclusion has also been described in parathyroid tumors,9,10 and it was noted in just a few cells in this case. That particular patient had a history of total thyroidectomy due to PTC, and there was no evidence of PTC recurrence.
Clinicoradiologic features and ancillary tests, such as immunohistochemical testing, can contribute to the correct diagnosis of PL.2,3,6 The sensitivity of cytological diagnosis varied substantially depending upon available clinicoradiologic information, such as serum PTH, ultrasonography, and location. However, the limits of clinicoradiologic information should also be considered. Nine of 22 patients showed a normal range of serum PTH in our study. An unusual location and other radiologic findings of PL can increase diagnostic difficulty. In our series, six of 25 PLs (24%) were intrathyroidal. This number is higher than the previously reported incidence of 53 of 4,868 (1%) parathyroid adenomas.11
In general, there are no distinguishing cytological features between parathyroid adenoma and hyperplasia.2,6 Therefore, it is recommended that PL is used in the routine cytological diagnosis if an aspirate suggests a parathyroid origin.2 However, a few studies have indicated that there might be distinguishing cytological features between parathyroid adenoma and hyperplasia.1,12,13
In conclusion, FNAC is a very useful method for the diagnosis of PL, although a few reports have mentioned the limitations of parathyroid FNAC, such as its low sensitivity, smear inadequacy, and contamination with thyroid tissue.3,8,13 On the basis of the findings from previous reports as well as our study, common cytological architectures of PL are scattered naked nuclei, loose clusters, prominent vasculature with epithelial cells resembling a papillary pattern, tight clusters, and a follicular pattern. These architectural features are frequently admixed at varying degrees. Common cellular features included anisokaryosis, stippled chromatin, a well-defined cell border, oxyphilic cytoplasm, and smaller nuclei than thyroid follicular cells. Minor features include intranuclear pseudoinclusions, colloid-like material, prominent nuclei, and eccentric nuclei. FNAC of the parathyroid can easily be confused with that of the thyroid because of their overlap in cytological features. Although no single cytomorphologic characteristic can be used as a diagnostic tool,2 a combination of cytological parameters and clinicoradiologic findings should be used to improve the diagnostic sensitivity of PL.
Notes
No potential conflict of interest relevant to this article was reported.