Cytomorphological Findings and Histological Correlation of Low-Grade Cribriform Cystadenocarcinoma of Salivary Gland in Fine-Needle Aspiration: A Case Study
Article information
Abstract
Low-grade cribriform cystadenocarcinoma (LGCCC) of the salivary gland is a rare tumor. We report the cytologic features and histologic correlation of a patient with LGCCC. A 57-year-old man had a hardly palpable, nontender mass in the right cheek area followed over nine months. Radiologic analysis revealed a 1.2 cm multiseptated, cystic, solid nodule in an anterior superficial lobe of the right parotid gland. Fine-needle aspiration cytology revealed many irregular overlapping sheets or clusters of ductal epithelial cells forming solid, pseudopapillary, and cribriform architectures. Nuclei of the tumor cells revealed inconspicuous atypia with minimal size variation. On the basis of these findings, we confirmed a diagnosis of ductal epithelial proliferative lesion, favoring neoplasm, with uncertain malignant potential. Tumor excision was performed, revealing a tiny multicystic nodule (0.7 cm). Histopathologically, this tumor showed the characteristic morphology of LGCCC. This is the first report of cytomorphological findings of LGCCC in Korea.
Low-grade cribriform cystadenocarcinoma (LGCCC) is a rare tumor defined as "a primary salivary duct tumor with clinically indolent behavior" in the 2005 World Health Organization (WHO) classification.1 This tumor was initially described as a "low grade salivary duct carcinoma" by Delgado et al.2 The characteristic histologic findings of this tumor were detailed in previously reported cases by several authors, and these findings included the absence of encapsulation, but a well-circumscribed, cystic, and cribriform pattern, resembling "low grade ductal carcinoma in situ or atypical ductal hyperplasia (ADH) pattern of breast".1-8 Clinically, salivary gland tumors are primarily aspirated, not excised. Although pathologists find fine-needle aspiration (FNA) cytology of salivary gland tumors difficult to diagnose, there is one previous report by Nakazawa et al.9 that describes the cytologic features of LGCCC. Here, we share our experience regarding the cytologic features and histologic correlation obtained by FNA of one case of LGCCC.
CASE REPORT
Brief patient history
A 57-year-old man had a barely palpable, nontender mass in the right cheek area, followed up over a nine-month period. Contrast-enhanced computed tomography revealed a mildly enhancing mass in an anterior superficial lobe of the right parotid gland (Fig. 1A). On ultrasonography, the lesion appeared as an approximately 1.2 cm large, multiseptated, cystic, solid nodule (Fig. 1B). FNA was performed and the cytologic examination led to a diagnosis of ductal epithelial proliferative lesion, favoring neoplasm, of uncertain malignant potential. Histologic confirmation was required.
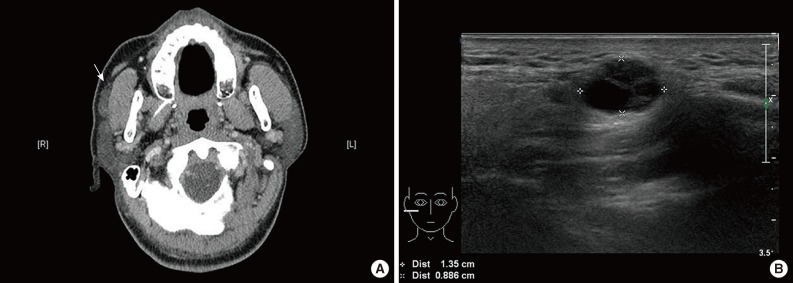
Radiologic findings. Enhanced computed tomography reveals a mildly enhanced mass (arrow) in the anterior superficial lobe of the right parotid gland. (A) It is separated from the main parotid gland and could be an accessory parotid gland tumor or lymphadenopathy. (B) On ultrasonography, an approximately 1.2-cm large, cystic, solid nodule is noted in the right cheek area.
Pathologic diagnosis
Excision of the lesion from the right parotid gland was performed. The surgical specimen revealed a tiny multicystic nodule (0.7 cm), which appeared to be surrounded by normal salivary gland tissue. The cut surface of the tumor was partly solid and partly cystic (Fig. 2A). Microscopically, it was a salivary ductal epithelial neoplasm showing multiple cysts lined by relatively bland ductal cells and a partly solid proliferation of ductal cells (Fig. 2B). Many of the superficial lining cells contained cytoplasmic apocrine-type microvacuoles that were periodic acid-Schiff-positive/diastase-resistant (Fig. 2C) and mucicarmine-positive (Fig. 2D). The tumor cells were strongly and diffusely S-100 protein positive (Fig. 2E). Myoepithelial markers (including actin and p63) highlighted the presence of cells surrounding the cystic spaces, confirming the intraductal nature of the lesion (Fig. 2F). No invasive foci were noted. On the basis of these findings, we diagnosed the mass as a low grade cribriform cystadenocarcinoma, marginally excised.
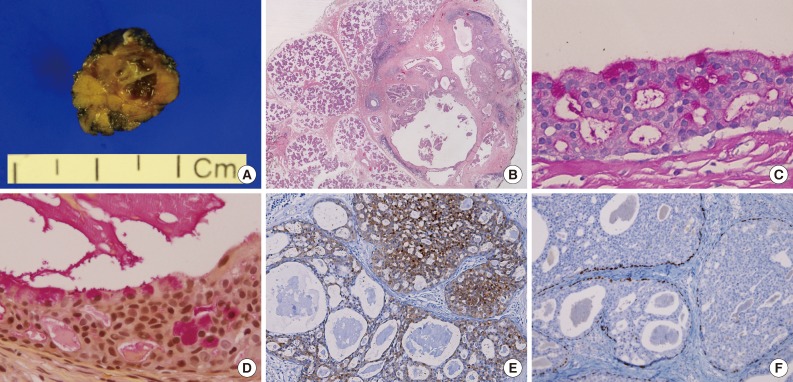
Gross findings and histopathology. (A) Gross findings reveal a tiny multicystic nodule (0.7 cm). (B) Microscopically, the tumor consists of multiple cysts with partly solid ductal epithelial proliferation. Many superficial cells contain cytoplasmic apocrine-type microvacuoles that are periodic acid-Schiff-positive/diastase-resistant (C) and mucicarmine-positive (D). (E) S-100 protein is diffusely expressed in the tumor cells. (F) p63 immunostaining demonstrates the presence of myoepithelial cells and absence of p63 in the intraductal proliferating tumor cells.
Cytomorphological findings and histological correlation
The cellular smear showed many large sheets or clusters of ductal epithelial cells in an irregular overlapping arrangement (Fig. 3A). These cytologic components could be aspirated from the solid area of this tumor (Fig. 3B). Architecturally, the tumor cells had tight intercellular connections forming a vague cribriform (Fig. 3C), solid, or pseudopapillary (Fig. 3E) arrangement. These were matched with histologic findings. The solid areas revealed a cribriform pattern, resembling ADH and low-grade ductal carcinoma in situ of the breast (Fig. 3D). The pseudopapillary projections were found in the epithelial lining the cystic areas (Fig. 3F). Cytologically, almost all tumor cells were bland looking, ductal epithelial cells with low nuclear/cytoplasmic (N/C) ratio. The nucleoli were found to be small, single, or entirely absent. Nuclear chromatin was fine or slightly coarse (Fig. 3G, H). Squamoid or metaplastic changes of the tumor cells, mentioned by Nakazawa et al.,9 were also identified in this case. Nuclear atypia was inconspicuous; however, one focus showed mild atypia with minimal nuclear size variation, prominent nucleoli, and vacuolated cytoplasm (Fig. 3I, J). The background was relatively clear in the cystic space. Scattered inflammatory cells with hemosiderin-laden macrophages, neutrophils, and eosinophils were seen. No evidence of necrosis or mucin was found.
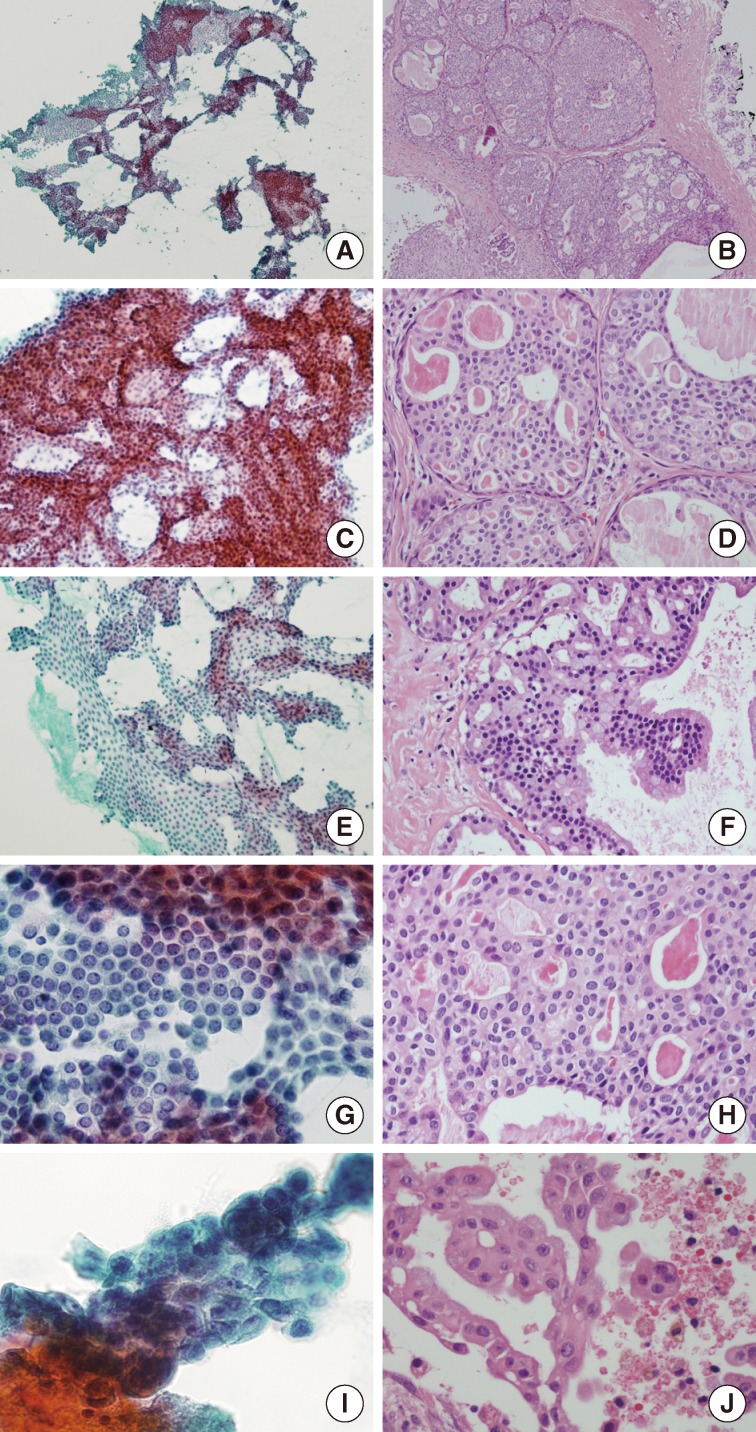
Cytomorphological findings and histological correlation. Papanicolaou staining of a smear reveals many sheets and clusters of ductal epithelial cells with irregular overlapping (A) which might be aspirated from solid areas of the tumor (B). Tight intercellular connections with vague cribriform arrangement (C), correlates with the histologic component of the solid area resembling atypical ductal hyperplasia and low-grade ductal carcinoma in situ of the breast (D). (E, F) The pseudopapillary architecture may be a micropapillary projection of the lining epithelium of cystic area. (G, H) Almost all tumor cells are bland-looking with low nuclear/cytoplasmic ratios, with inconspicuous or one small nucleolus and relatively fine nuclear chromatin. (I, J) Only small foci of tumor cells have mild cytologic atypia with minimal size variation and prominent nucleoli.
Postoperative outcome
Whole-body positron emission tomography-computed tomography scanning was conducted after surgery. A residual, enhancing nodular lesion in the anteriosuperior aspect of the right parotid gland showed mild, increased fluorodeoxyglucose uptake. Overall, residual malignancy was suggested. Postoperative chemotherapy or radiation therapy was not recommended. A follow-up was conducted two months later. At that time, the patient was healthy, with no evidence of recurrence or distant metastasis.
DISCUSSION
This is the first report of the cytomorphological findings of LGCCC in Korea and the second report in the English literature. Compared with the first case, described by Nakazawa et al.,9 the two cases share common cytomorphology summarized as follows: 1) irregular and overlapping sheets or clusters of ductal epithelial proliferation with tight connections; 2) mild nuclear atypia with low N/C ratio, slightly coarse chromatin, inconspicuous or one small nucleolus and minimal size variation; 3) cytoplasmic vacuoles and squamoid or metaplastic changes of tumor cells; and 4) a cystic or inflammatory background with mixed inflammatory cells and hemosiderin-laden macrophages with no evidence of necrosis or mucin. Further, we report the following new findings that were not reported by Nakazawa et al.9: 1) tumor cells with solid, pseudopapillary, or cribriform architectural arrangement; 2) absence of "large yellow to brown pigments" in the cytoplasm of the tumor cells; 3) the smear in our case showed more benign-looking cytomorphology, with almost all tumor cells presenting inconspicuous cytologic atypia, resembling instead benign, ductal proliferating cells. Only a few clusters of tumor cells revealed minimal cytologic atypia.
Nakazawa et al.9 presented salivary duct carcinoma, papillary cystic variant of acinar cell carcinoma, and mucoepidermoid carcinoma as the results of differential diagnoses. However, in this case, we would consider benign salivary gland adenoma as the differential diagnosis since almost all tumor cells showed benign-looking cytomorphology. However, this diagnosis can be ruled out by the small clusters of mild cytologic atypia, as previously described. Adenoid cystic carcinoma (ACC) might be considered a differential diagnosis due to similar architecture at low magnification; however, the cribriform architecture of LGCCC is different from ACC. It can be described as "less defined" or "more vague" than ACC because basaloid cells were not identified in the cribriform area. Typical hyaline globules were also not seen in our case. Finally, the cytologic atypia observed in this case was too mild. High-grade tumors can also be ruled out as a diagnosis because the observed atypia was not severe or marked; rather, it was very minimal or mild.
In conclusion, when pathologists are presented with an aspiration cytology of salivary gland tumors, an effort must be made to discover any small foci showing minimal atypism; otherwise, there is potential to overlook the possibility of a low-grade malignancy such as LGCCC. We recommend more intensive investigation of FNA specimens.
This report is important as it is the second case report in the English literature on the cytologic features of LGCCC in FNA. Herein, we also described histologic correlations, which provide new observations, and the absence of previously reported features. Furthermore, we hope to encourage more authors to report cytomorphologic features of LGCCC in salivary gland in order to gather diagnostic criteria in FNA of this tumor. Until then, when LGCCC is suspected in FNA cytology, an improved descriptive diagnosis will facilitate the differential diagnosis and the recommendation of surgical excision, which would be preferred over a report limited only to a specific diagnosis.
Notes
No potential conflict of interest relevant to this article was reported.